pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Narang Hong,Hyung Don Kook,Dong Heon Lee,Jiyoung Ahn,Mi Youn Park,Hye Jung Jung
10.17966/JMI.2022.27.1.19 Epub 2022 April 02
Abstract
PATIENT CONSENT STATEMENT: The patient provided written informed consent for the publication and the use of her images.
Keywords
COVID-19 vaccine mRNA vaccine Pilomatricoma
The United States' Food and Drug Administration had authorized the use of the COVID-19 vaccine since 2020 due to the COVID-19 pandemic. Vaccines developed by Pfizer/ BioNTech, and Moderna are unique mRNA vaccines with different manufacturing processes, among the different types of vaccine platforms.
As vaccination rates have increased, cutaneous adverse events have been reported. Injection site reaction was the most common adverse event, followed by urticaria, morbilli- form eruptions, and aggravation of existing skin diseases1. While similar events have been reported with other vaccines, there have been no reports of unique adverse events, such as skin symptoms similar to those seen in COVID-19 patients or skin lesions that may appear at the hyaluronic filler injection site1. In this paper, we describe a case of pilomatricoma that developed after receiving the COVID-19 vaccine.
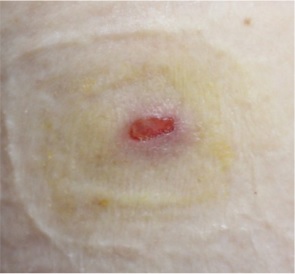
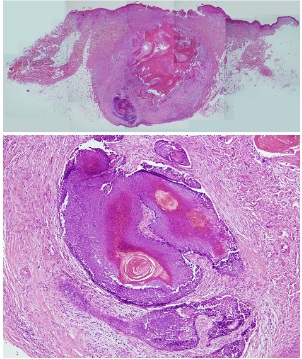
A nodule on the left upper arm of a 76-year-old woman was noticed. Two months prior to consultation, the patient received a COVID-19 vaccine injection in the left upper arm (ComirnatyTM Injection, Pfizer/BioNTech). Pain and edema remained at the site for a long time after that. She reported seeing clinical signs of infection at the site three weeks later, at her second inoculation visit. Therefore, she received anti- biotics and a second inoculation in her right arm. However, there was no improvement. There was an indurated sub- cutaneous nodule in the left upper arm when she came to our hospital, with erosion on the overlying epidermis (Fig. 1). A biopsy revealed a well-circumscribed tumor within the dermis after an excisional procedure (Fig. 2). Basaloid cells were found in the tumor's periphery, while ghost cells were found in the center. Additionally, giant cells were discovered, which are transitional cells that represent the process of a basaloid cell becoming a shadow cell. The patient was diag- nosed with pilomatricoma based on histological findings. Her pilomatricoma lesion was removed after the biopsy.
Pilomatricoma is a benign tumor of the head and neck that develops from hair matrix cells and presents as an asymp- tomatic subcutaneous nodule2. However, the pathogenesis of pilomatricoma is not yet fully understood. After trauma, approximately 9% of patients have been reported to develop pilomatricoma (e.g., insect bite, vaccine, etc.). It's also been linked to subcutaneous methotrexate injections and vaccina- tions like BCG, tetanus, hepatitis A, and influenza2-5. The assumptions regarding the mechanism of pilomatricoma after trauma are as follows: Needlestick injury damages the fol- licular epithelium, resulting in faulty apoptosis suppression and the formation of pilomatricoma. Inflammation can be aggravated by the attenuated agent itself or by adjuvants in vaccines2-5. However, needlestick injury itself is a more important trigger of pilomatrioma, because there are reported cases of pilomatricoma developing after injection of drugs other than the vaccine2-5. After inoculation, the patient's inflammation persisted, with lymphocytes and giant cells infiltrating the area. Although this is not the first reported case of pilomatricoma developing after receiving the vaccine, it is the first case of pilomatricoma developing after receiving an mRNA vaccine, to the best of our knowledge.
References
1. McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol 2021;85:46-55
Google Scholar
2. Hernández-Núñez A, Nájera Botello L, Romero Maté A, Martínez-Sánchez C, Utrera Busquets M, Calderón Komáromy A, et al. Retrospective study of pilomatricoma: 261 tumors in 239 patients. Actas Dermosifiliogr (English Edition) 2014;105:699-705
3. Afonso Pérez E, Garcia Porrua C, Castiñeiras Mato IM, Bal Nieves F. Development of pylomatrixoma after the sub- cutaneous injection of methotrexate for the treatment of juvenile idiopathic arthritis. Rheumatol Clin (English Edition) 2012;8:380-381
Google Scholar
4. Aquilina S, Gatt P, Boffa MJ. Pilomatricoma arising at a BCG vaccination site. Clin Exp Dermatol 2006;31:296-297
Google Scholar
5. Pirouzmanesh A, Reinisch JF, Gonzalez-Gomez I, Smith EM, Meara JG. Pilomatrixoma: a review of 346 cases. Plast Reconstr Surg 2003;112:1784-1789
Google Scholar
Congratulatory MessageClick here!