pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Kyung Jae Lee ,Jung Eun Kim
10.17966/JMI.2021.26.4.87 Epub 2022 January 01
Abstract
Background: Several dermoscopic findings that could be helpful in diagnosing onychomycosis have been reported in many cases, but they have not been sufficiently utilized in clinical practice.
Objective: To evaluate and identify the dermoscopic findings that may assist in the accurate diagnose of onychomycosis.
Methods: The study included 42 patients with clinical features suggestive of onychomycosis based on the clinical history, physical examination, dermoscopic findings, and mycological investigation. Clinical photographs and nail dermoscopy images were obtained, which were retrospectively reviewed and analyzed according to the onychomycosis classification.
Results: In total, 42 representative nails were reviewed. Common dermoscopic patterns such as yellow/brown discoloration and subungual hyperkeratosis were found in our onychomycosis patients. Key findings observed in specific subtypes were distolateral subungual onychomycosis with "jagged edges with spikes", proximal subungual onychomycosis or white superficial onychomycosis with irregularly bordered homogeneous leukonychia with postinflammatory hyperpigmentation on the proximal nail fold, and fungal melanonychia with nail plate roughness and nail fold hyperkeratosis.
Conclusion: Our study, along with previous studies, demonstrated dermoscopy as a quick and effective tool for diagnosing onychomycosis. In addition, periungual dermoscopic findings can be an important clue in onychomycosis diagnosis, especially in cases of fungal melanonychia and leukonychia.
Keywords
Fungal Dermoscopy Onychomycosis
Onychomycosis is a fungal nail infection caused by dermatophytes, nondermatophytic molds, or yeasts. Although its clinical features are relatively obvious, at least one con- firmatory test is required to avoid unnecessary treatment. Treatment is often systemic and long-term; consequently, this increases the risk of adverse effects. The most common diagnostic confirmatory tests for onychomycosis include direct microscopy with potassium hydroxide (KOH), fungal culture, polymerase chain reaction, and histopathological examination using periodic acid-Schiff staining1.
Dermoscopy is a widely used, non-invasive, and easily available diagnostic method for many dermatological dis- eases1-3. Recently, several dermoscopic patterns useful in the diagnosis of onychomycosis have been identified. Piraccini et al. described the characteristic dermoscopic patterns in distolateral subungual onychomycosis (DLSO)4, and Kaynak et al. reported characteristic dermoscopic patterns that may aid in its diagnosis1. Meanwhile, Kayarkatte et al. described specific onychoscopic features for different clinical variants of onychomycosis5.
In this study, we evaluated common onychomycosis dermoscopic findings and patterns helpful in the diagnosis of onychomycosis and analyzed them according to the onychomycosis classifications.
1. Data collection
1) Study type
This is a cross-sectional observational study conducted at Eunpyeong St. Mary's Hospital from November 2018 to January 2021. The study was exempted from the approval requirement by the Catholic University of Korea Eunpyeong St. Mary's Hospital ethics committee (IRB no. 2021-1689-0001), and informed consent was obtained from the patients for the use of nail photography included in this study. All patients' medical records and clinical photographs were reviewed retrospectively.
2) Study participants
We recruited 42 adult patients with features suggestive of onychomycosis. Patients with non-infectious pre-existing diseases contributing to nail pathology or those who had received topical or systemic antifungal medications within 1~3 months from the first day of visit were excluded.
3) Clinical examination
A picture of the patient's onychomycotic nails was taken and saved in JPEG format.
The clinical onychomycosis subtypes included in this study are DLSO, proximal subungual onychomycosis (PSO), white superficial onychomycosis (WSO), total dystrophic onycho- mycosis (TDO), and fungal melanonychia (FM).
4) Nail dermoscopy (onychoscopy)
A single representative nail was selected from each patient for nail dermoscopy. Using a DermLite Cam®dermoscope (3Gen, San Juan Capistrano, CA, USA), each patient's der- moscopic nail image was taken at 10x magnification.
Definitions of the dermoscopic patterns analyzed in our study are described in Table 1.
|
Dermoscopic
pattern |
Definition |
|
Subungual hyperkeratosis |
Accumulation of scales under the
distal portion of the nail plate, with nail thickening and uplifting |
|
Jagged edge with spikes |
Jagged edges are seen in proximal
margin of the onycholytic area, with sharp whitish longitudinal lines
extending proximal to the nail plate |
|
Transverse striae |
Horizontal lines parallel to the
lunula, with colors ranging from white to brown |
|
Distal irregular termination |
Irregular ending of the thickened
nail plate with crumbly appearance |
|
Yellow discoloration |
Color changes ranging from light
yellow to orange |
|
Brown discoloration |
Color changes ranging from light
brown to dark brown |
|
Black discoloration |
Black color change except for the
bleeding and artificial color changes in the nail plate |
|
Homogeneous leukonychia |
White color changes greater than 1
mm on the nail plate |
|
Longitudinal leukonychia |
White longitudinal lines parallel
to the grooves on the nail plate |
|
Punctate leukonychia |
White spots or flecks with
dimensions of less than 1 mm on the nail plate |
|
Nail plate destruction (=onychodystrophy) |
Partial or complete disruption of
the various keratinous layers of the nail plate |
|
|
|
5) Sample collection for direct microscopic examination with 20% KOH
All specimens were collected through subungual debris and nail plate scraping according to the clinical subtypes of onychomycosis.
Samples were collected by subungual debris in DLSO and FM, and nail plate scraping in PSO and WSO. To minimize contamination, specimens were collected from the most proximal affected area of the representative nail without causing trauma.
Direct microscopic examination with 20% KOH was not performed in cases wherein the clinical signs were apparent.
2. Statistical analysis
Data were coded and entered into Microsoft Excel. The proportions of each dermoscopic pattern were compared.
A total of 42 suspected onychomycosis patients with dermoscopic nail images were enrolled. Of the 42 patients, 18 had onychomycosis as confirmed through KOH smears. Meanwhile, 24 patients had relatively apparent clinical evi- dence of onychomycosis at the first visit and showed clean nail growth on the proximal nail plate after topical efinaconazole or amorolfine treatment during outpatient follow-up. In this study, the dermoscopic images of the 42 representative nails were reviewed.
1. Common dermoscopic findings in onycho- mycosis
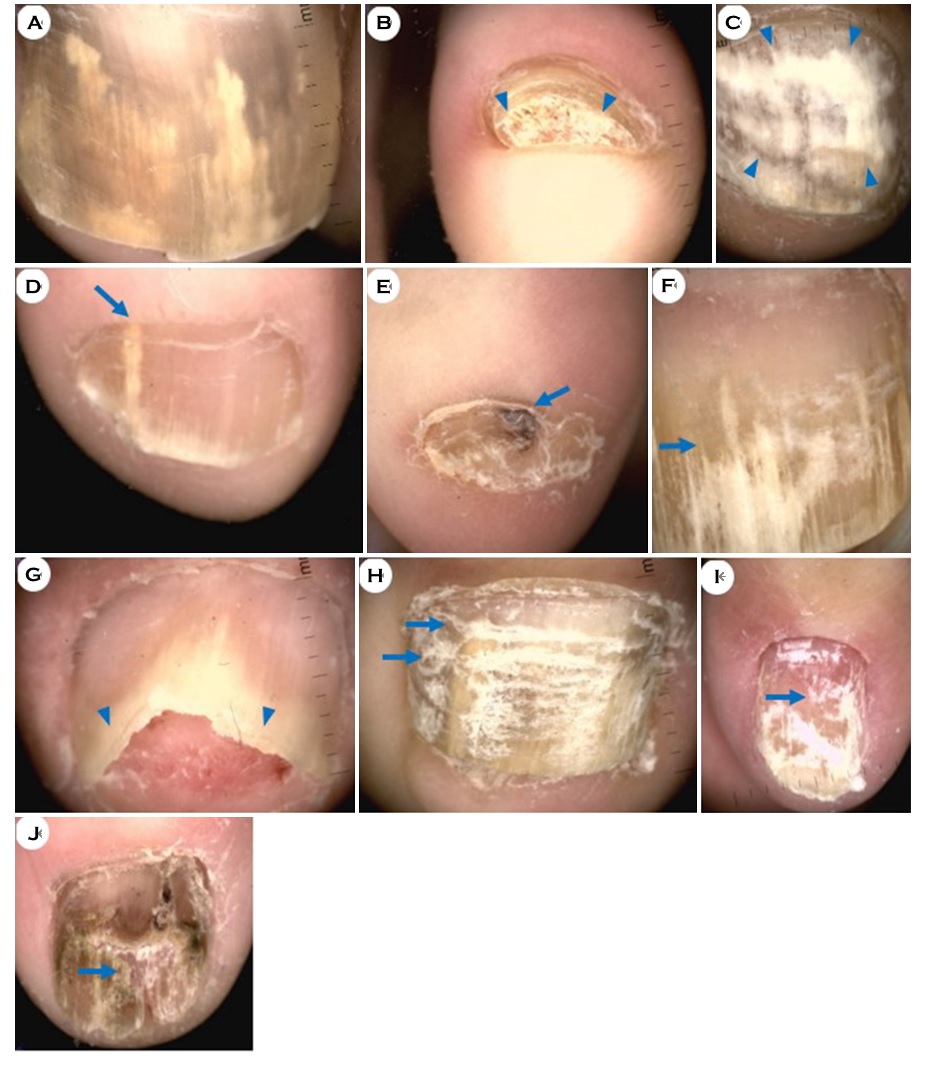
Dermoscopy images of each representative nail were taken and collected, and the dermoscopic patterns observed in each image were recorded. Thereafter, we analyzed the number and proportion of specific onychomycosis dermoscopic pat- terns (Fig. 1).
Color change of the nail plate, such as yellow/brown dis- coloration, was the most frequently observed feature. Yellow and brown discolorations were seen in 52.4% and 50.0% nails, respectively (Fig. 1A). Subungual hyperkeratosis (Fig. 1B), homogeneous leukonychia (Fig. 1C), longitudinal leukonychia (Fig. 1D), black discoloration (Fig. 1E), jagged edge with spikes (Fig. 1F), distal irregular termination (Fig. 1G), trans- verse striae (Fig. 1H), punctate leukonychia (Fig. 1I), and nail plate destruction (Fig. 1J) were observed in decreasing order (Table 2).
|
Description |
Number |
Percentage |
|
Yellow
discoloration |
22 |
52.4 |
|
Brown
discoloration |
21 |
50.0 |
|
Subungual
hyperkeratosis |
20 |
47.6 |
|
Homogeneous
leukonychia |
14 |
33.3 |
|
Longitudinal
leukonychia |
10 |
23.8 |
|
Black
discoloration |
10 |
23.8 |
|
Jagged
edge with spikes |
9 |
21.4 |
|
Distal
irregular termination |
8 |
19.0 |
|
Transverse
striae |
3 |
7.1 |
|
Punctate
leukonychia |
3 |
7.1 |
|
Nail
plate destruction (=onychodystrophy) |
3 |
7.1 |
|
|
||
2. Dermoscopic findings according to the clinical subtypes of onychomycosis
The samples in our study were classified according to the clinical subtypes as summarized in Table 3. DLSO was the most frequently observed clinical subtype (34/42; 80.9%), followed by FM (3/42; 7.1%), WSO (2/42; 4.8%), TDO (2/42; 4.8%), and PSO (1/42; 2.4%) (Table 3). The characteristic onychoscopy features of each clinical subtype were observed in this study (Figs. 2-4).
|
Subtype |
Number |
Percentage |
|
Distolateral subungual |
34 |
80.9 |
|
Fungal melanonychia |
3 |
7.1 |
|
White superficial |
2 |
4.8 |
|
Total dystrophic |
2 |
4.8 |
|
Proximal subungual |
1 |
2.4 |
|
|
||
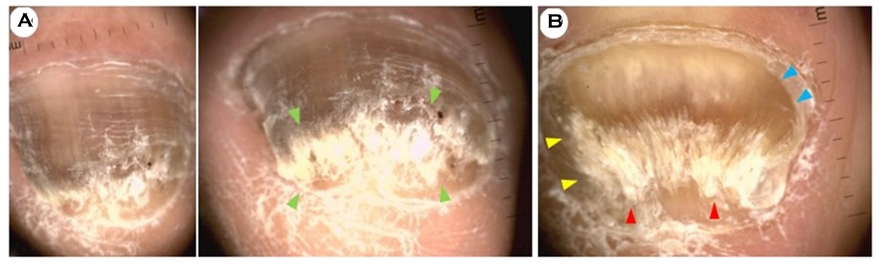
The onychoscopic pattern in DLSO, which accounted for the largest proportion, showed brownish discoloration of the nail plate and a ruined appearance (subungual hyperkeratosis)(Fig. 2A). The onychoscopic pattern of FM showed total melanonychia and mild subungual hyperkeratosis, accompanied by the pseudo-Hutchinson sign (Fig. 4B). Here the pseudo-Hutchinson sign was defined as nail matrix pigmentation detected through a relatively translucent cuticle at the proximal nail fold. The onychoscopic pattern in TDO showed onychodystrophy, subungual hyperkeratosis, and brownish discoloration (Fig. 2B). The onychoscopic pattern in PSO showed homogeneous leukonychia of the proximal nail plate (Fig. 3A). The patients had no previous history of immuno- compromizing diseases such as HIV. In WSO, there was homogeneous leukonychia on most of the nail plate (Figs. 3B, 3C).
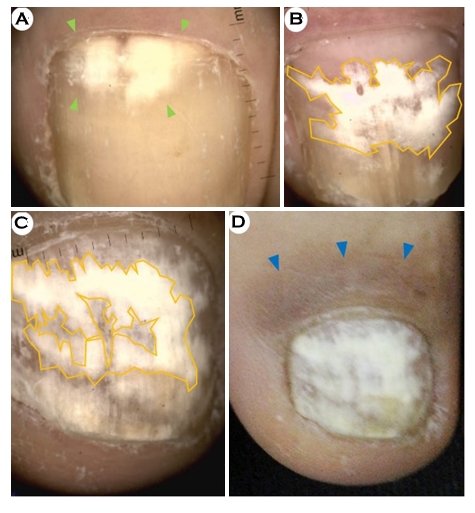
3. Dermoscopic findings of fungal leukonychia
In our study, the proportion of nails with leukonychia was 64.3% (27/42). We further subdivided the leukonychia samples and classified them according to their morphological type as follows: homogeneous leukonychia (14/42; 33.3%) (Fig. 1C and Fig. 3); longitudinal leukonychia (10/42; 23.8%) (Fig. 1D); and punctate leukonychia (3/42; 7.1%) (Fig. 1I).
The homogeneous leukonychia pattern was commonly observed in PSO and WSO cases (Fig. 3) and was also seen in several DLSO cases (10/31; 32.3%).
In this study, a specific pattern of fungal leukonychia was observed in the WSO subtypes, which was homogeneous leukonychia with irregular borders (Figs. 3B and 3C, magnification, ×10). Severe post-inflammatory hyperpigmentation was observed in the proximal nail fold, which was considered to have resulted from the id reaction to the fungal infection (Fig. 3D, clinical view).
4. Dermoscopic findings related to fungal melanonychia
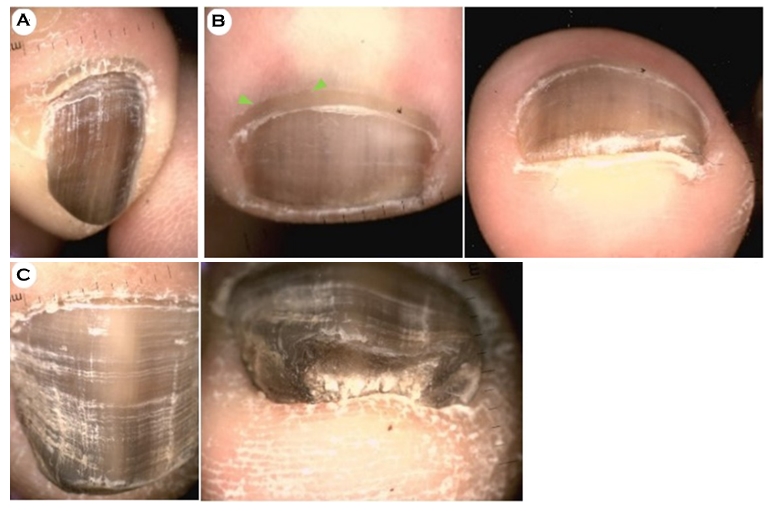
Three patients with FM were included in this study (Fig. 4), and the nails of all three showed dark-brownish, diffuse, and asymmetric pigmentations with a positive KOH test (Fig. 4A-C). In addition, one case showed the pseudo-Hutchinson sign (Fig. 4B), and two cases were accompanied by subungual hyperkeratosis, which was prominently observed in the frontal view during dermoscopy (Fig. 4B-C).
As nail unit melanoma was a differential diagnosis, all patients were followed up while receiving systemic antifungal treatment, and all showed clinical improvement in terms of melanonychia.
Five onychomycosis clinical subtypes were observed in this study. DLSO comprised the majority of cases with 80.9%, followed by FM (7.1%), WSO (4.8%), TDO (4.8%), and PSO (2.4%). These findings are consistent with those of a dermoscopic study conducted by De Crignis et al. (DLSO in 66.93%) covering a total of 502 case series6.
The most frequently observed dermoscopic pattern in our study was nail plate color change. Yellow and brown dis- colorations were noted in 52.4% and 50% of cases, respectively, which was more than half of all onychomycosis cases, followed by black discoloration (23.8%). A similar pattern of nail color change was also reported by Piraccini et al. (yellow > brown > black: 70% > 51% > 24%)4. In their study, 59% of cases showed a whitish color change, which was statistically significant in the diagnosis of onychomycosis. In previous studies, white discoloration of the nail bed or plate was described in various ways such as opacity, white chromonychia, and leukonychia4,6.
Leukonychia is classified into three subtypes based on its pathologic origin: true leukonychia (pathology originates in the matrix and emerges in the nail plate), apparent leukonychia (pathology is in the nail bed), and pseudoleukonychia (nail plate pathology is exogenous i.e., onychomycosis)7. Leuk- onychia associated with systemic disease tends to be true or apparent, and shows characteristic leukonychia patterns7. In our cases, whitish nail color changes were consistent with pseudoleukonychia (Fig. 3).
Kayarkatte et al. reported that the specific morphological types of leukonychia were not statistically significant in the diagnosis of onychomycosis5. However, in the present study, postinflammatory hyperpigmentation of the proximal nail fold along with leukonychia or leukonychia with irregular borders and various chroma was found in the fungal leukonychia cases. Irregular borders of leukonychia may result from fungal invasion and subungual hyperkeratosis. Postinflammatory hyperpigmentation was commonly seen in PSO or WSO cases, which is thought to be due to inflammatory responses to the fungus in the proximal nail fold.
In cases where the clinical features of leukonychia are characteristically expressed, such as fungal balls, the diagnosis of onychomycosis is not difficult. However, in cases where the cause of leukonychia is not clear, the dermoscopic findings can be a decisive clue.
In this study, subungual hyperkeratosis was the most common pattern among the nail changes (47.6%; 20/42). These dermoscopic patterns were observed in 45.2% of DLSO cases (14/31). These findings are consistent with the study by Rathod et al. in which subungual hyperkeratosis was present in 73.9% of all the DLSO cases8.
A study by Kaynak et al., which was conducted on 205 nail samples from 97 DLSO patients, observed that 92.7% of samples had a ruined appearance pattern, and logistic regression model analysis revealed that this pattern was statistically significant in the diagnosis of DLSO (p = 0.015)1. However, as described above, the proportion of subungual hyperkeratosis in our study was only 47.6%, which was thought to be due to the differences in infection severity and disease duration.
In the present study, the second most frequently observed nail shape change was the "agged edge with spike" patterns (9/42; 21.4%) (Fig. 1F). All of the "spikes" patterns were observed in DLSO only; in 29.0% (n = 9) of all the DLSO cases. These findings were consistent with a study by Abdallah et al., which reported that "jagged proximal edges" and "intermittent spiked" patterns were more common in DLSO and TDO9.
Piraccini et al. suggested that "jagged proximal edge with spikes" were exclusive to DLSO4. The jagged edge results from the proximal progression of dermatophytes along with the horny layer of the nail bed longitudinal ridges, while the matte discoloration (homogeneous white, yellow, and brown color) reflects the color of the colonies, scales, and subungual debris4.
The dermoscopic findings of the longitudinal spikes may be found in brittle nail syndrome, also known as onychorrhexis6. However, they are not observed on the nail surface and usually start from the proximal region going toward the distal region. This is different from that of onychomycosis longitudinal spikes in DLSO, which are located on the subungual region and start from the distal portion of the nail plate.
FM is a rare cause of nail pigmentation. Diagnosing FM may be very challenging because it can easily be confused clinically with melanocyte related melanonychia10. The etiology of melanonychia is multifactorial, with causes that include subungual hematoma, fungal infection, ethnic type pigmentation, drug- or trauma-induced pigmentation, lentigo, nevus, and malignant melanoma11.
To date, there are very few research studies on the dermoscopic findings of FM. Previous studies described dermoscopic findings of 14, 18, 33, and 20 FM patients10,12-14. Kilinc Karaarslan et al. suggested that multicolored pigmen- tation and reverse triangular patterns (pigmentation that was wider at the distal than the proximal end) are distinctive features of FM; however, their study was limited by its small sample size and the absence of a control group. Ohn et al. compared the dermoscopic findings of FM with those of other causal melanonychias such as nail matrix nevus, melanocytic activation of the nail matrix, and malignant melanoma. Their study reported that several dermoscopic features, such as reverse triangular patterns, subungual hyperkeratosis, and white or yellow streaks, were characteristic of FM. In particular, subungual keratosis increased the likelihood of FM by about 14-fold. Similarly, in our study, 66.7% of the FM cases accompanied subungual hyperkeratosis. Based on our findings, nail plate roughness is an additional FM feature, and it can be more helpful in the diagnosis of FM when accompanied by proximal nail fold hyperkeratosis (Fig. 4A).
Elmas et al. evaluated each of the lesions in combination with the pigmentation color and pattern12. The most common presentation was homogeneous brown pigmentation (15/42; 35.7%), and a pseudo-Hutchinson sign was observed in 9.5% (4/42) of lesions. Similarly, in our present study, all 3 FM cases showed dark-brownish homogeneous pigmentations (3/3; 100%), and a pseudo-Hutchinson sign was observed in 33.3% (1/3). However, continuous follow-up is important to differentiate it from a pseudo-Hutchinson sign the true Hutchinson sign.
Our study included some participants who were diagnosed only clinically and not confirmed by mycological examination. Although we used the data of those who only showed clinical improvement after topical antifungal treatment, it is a limitation of our study.
Another limitation of our study is that we did not compare the dermoscopic findings with those of normal controls or controls with non-infectious nail disorders. Our study was also limited by its small sample size, so further large-scale studies are required to validate these findings.
We found that dermoscopic patterns such as yellow/brown discoloration and subungual hyperkeratosis were common in our onychomycosis patients. In addition, the characteristic dermoscopic pattern "jagged edge with spikes" was observed in DLSO subtypes.
Our study, along with previous studies, showed that dermoscopy can be a quick and effective tool for diagnosing onychomycosis. In addition, periungual dermoscopic findings can be important clues when diagnosing onychomycosis, especially in cases of FM and leukonychia.
References
1. Kaynak E, Goktay F, Gunes P, Sayman E, Turan D, Baygul A, et al. The role of dermoscopy in the diagnosis of distal lateral subungual onychomycosis. Arch Dermatol Res 2018;310:57-69
Google Scholar
2. Kato J, Horimoto K, Sato S, Minowa T, Uhara H. Der- moscopy of melanoma and non-melanoma skin cancers. Front Med (Lausanne) 2019;6:180
3. Weber P, Tschandl P, Sinz C, Kittler H. Dermatoscopy of neoplastic skin lesions: recent advances, updates, and revisions. Curr Treat Options Oncol 2018;19:56
Google Scholar
4. Piraccini BM, Balestri R, Starace M, Rech G. Nail digital dermoscopy (onychoscopy) in the diagnosis of onycho- mycosis. J Eur Acad Dermatol Venereol 2013;27:509-513
Google Scholar
5. Kayarkatte MN, Singal A, Pandhi D, Das S, Sharma S. Nail dermoscopy (onychoscopy) findings in the diagnosis of primary onychomycosis: a cross-sectional study. Indian J Dermatol Venereol Leprol 2020;86:341-349
Google Scholar
6. De Crignis G, Valgas N, Rezende P, Leverone A, Nakamura R. Dermatoscopy of onychomycosis. Int J Dermatol 2014; 53:e97-99
Google Scholar
7. Singh G. Nails in systemic disease. Indian J Dermatol Venereol Leprol 2011;77:646-651
Google Scholar
8. Rathod D, Makhecha MB, Chatterjee M, Singh T, Neema S. A cross-sectional descriptive study of dermoscopy in various nail diseases at a tertiary care center. Int J Dermato 2017;1:11-19
9. Abdallah NA, Said M, Mahmoud MT, Omar MA. Onycho- mycosis: correlation between the dermoscopic patterns and fungal culture. J Cosmet Dermatol 2020;19:1196-1204
Google Scholar
10. Ohn J, Choe YS, Park J, Mun JH. Dermoscopic patterns of fungal melanonychia: a comparative study with other causes of melanonychia. J Am Acad Dermatol 2017;76: 488-493. e2
Google Scholar
11. Haneke E, Baran R. Longitudinal melanonychia. Dermatol Surg 2001;27:580-584
Google Scholar
12. Elmas ÖF, Metin MS. Dermoscopic findings of fungal melanonychia. Postepy Dermatol Alergol 2020;37:180-183
Google Scholar
13. Kilinc Karaarslan I, Acar A, Aytimur D, Akalin T, Ozdemir F. Dermoscopic features in fungal melanonychia. Clin Exp Dermatol 2015;40:271-278
Google Scholar
14. Kim HJ, Kim TW, Park SM, Lee HJ, Kim GW, Kim HS, et al. Clinical and dermoscopic features of fungal melanonychia: differentiating from subungual melanoma. Ann Dermatol 2020;32:460-465
Google Scholar
Congratulatory MessageClick here!