pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Taekwoon Kim,Jeongsoo Lee,Joonsoo Park
10.17966/JMI.2021.26.3.65 Epub 2021 October 04
Abstract
Dermatomycosis is a skin disease caused by fungi, including dermatophytes and yeasts. Its diagnostic methods include KOH smear, fungal culture test, Wood's lamp test, biopsy, and molecular biology test. Superficial dermatomycosis can already be diagnosed using only KOH smear and culture test, so biopsy has not yet received attention from many clinicians. Nonetheless, biopsy is one of the most basic tests in the field of dermatology, with high diagnostic value for deep and superficial dermatomycoses, which often shows negative findings on KOH smear or fungal culture test. In this study, the histopathologic findings and special chemical staining methods in dermatomycosis are described. This review article is an upgraded English version of the review paper "Dermatomycosis from the perspective of dermatopathology (Korean Version 1.0)" published in 2016.
Keywords
Fungal infection Histopathology Special stain
Despite the clinical significance of dermatopathology in the field of dermatomycosis, there are still limited studies about this subject. Therefore, as a response to a number of requests to upgrade and publish the study "Dermatomycosis from the perspective of dermatopathology (Version 1.0)" in English, Version 1.1 (in English) is described in this review article.
Dermatomycosis is a skin disease caused by fungi. In general, it is divided into superficial dermatomycosis and deep dermato- mycosis. Superficial dermatomycosis invades the epidermis and skin appendages, while deep dermatomycosis invades the dermis and subcutaneous fat layer1. The diagnostic methods for skin mycoses include KOH smear, fungal culture test, Wood's lamp test, biopsy, and molecular biology test2. Among these, biopsy is widely used to diagnose deep dermato- mycosis. However, superficial dermatomycosis can already be diagnosed using only KOH smear and culture test, so biopsy has not yet received attention from many clinicians.
Nonetheless, biopsy is one of the most basic tests in the field of dermatology, and its diagnostic value is high for deep and superficial dermatomycoses. In fact, patients with superficial dermatomycosis often show negative findings on KOH smear or fungal culture test, and it is easy to overlook cutaneous mycoses when clinically nonspecific lesions are formed3. Examples include tinea incognito caused by prior treatments, such as local steroid ointment, and Majocchi granulomas that occur in the dermis or subcutaneous tissue due to the causative agent of superficial dermatomycosis. In these cases, as the typical clinical manifestations of superficial dermatomycosis are not observed, its diagnosis is delayed or misdiagnosed as another skin disease, resulting in the repetition of wrong treatments. For these cases, the diagnosis can be made through biopsy. When skin biopsy is per- formed, the diagnosis is made by confirming the presence of hyphae or spores, which are structures peculiar to fungi in tissue sections. If there is no knowledge and recognition about this, fungal infections may be overlooked4. In this study, the biopsy findings and chemical staining methods for dermatomycosis are described.
HISTOLOGICAL EXAMINATION OF DERMATOMYCOSIS
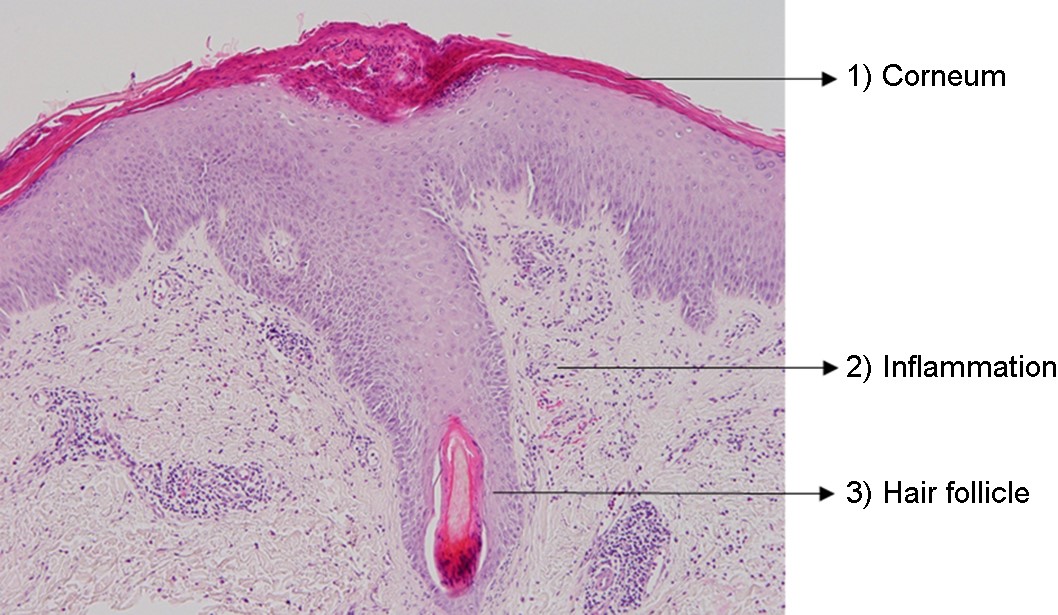
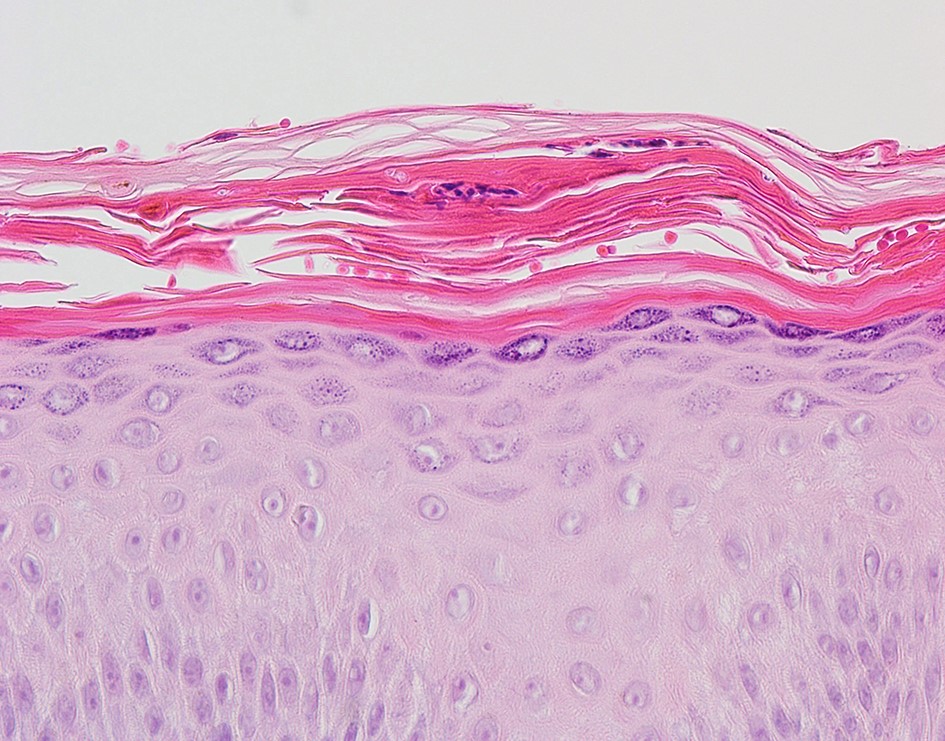
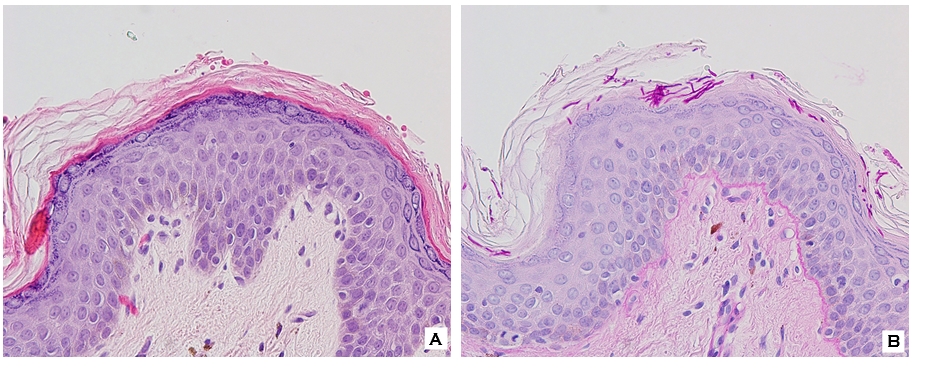
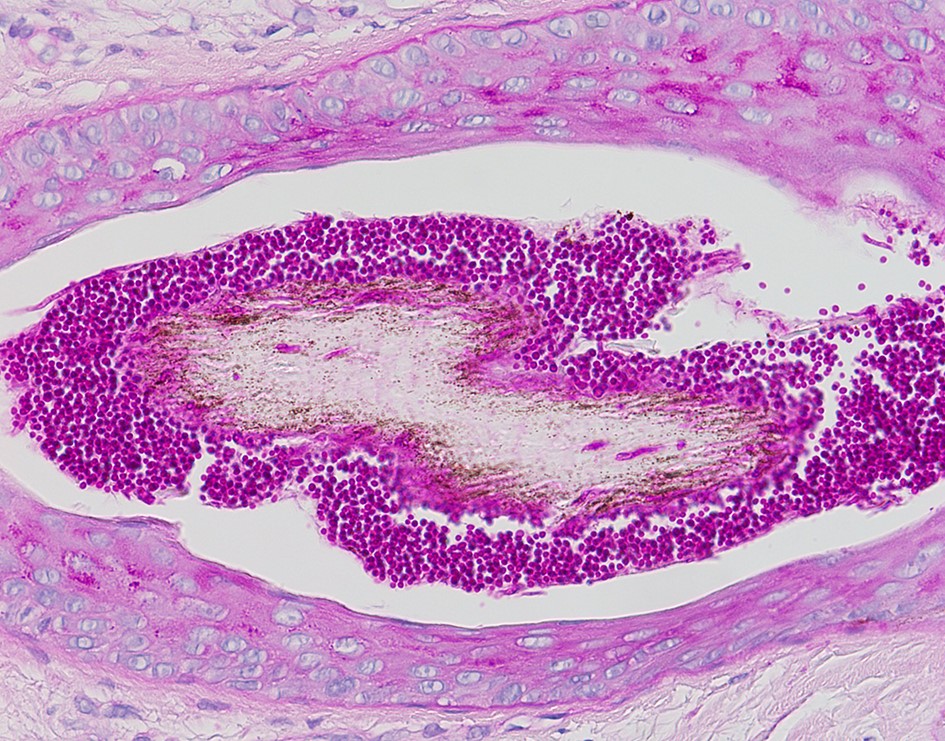
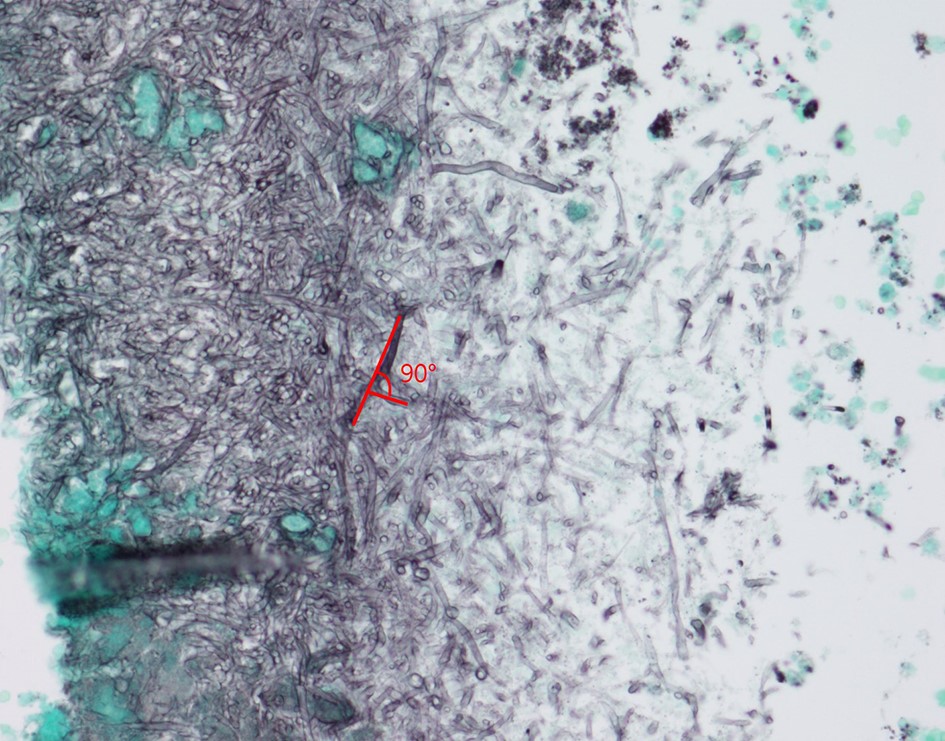
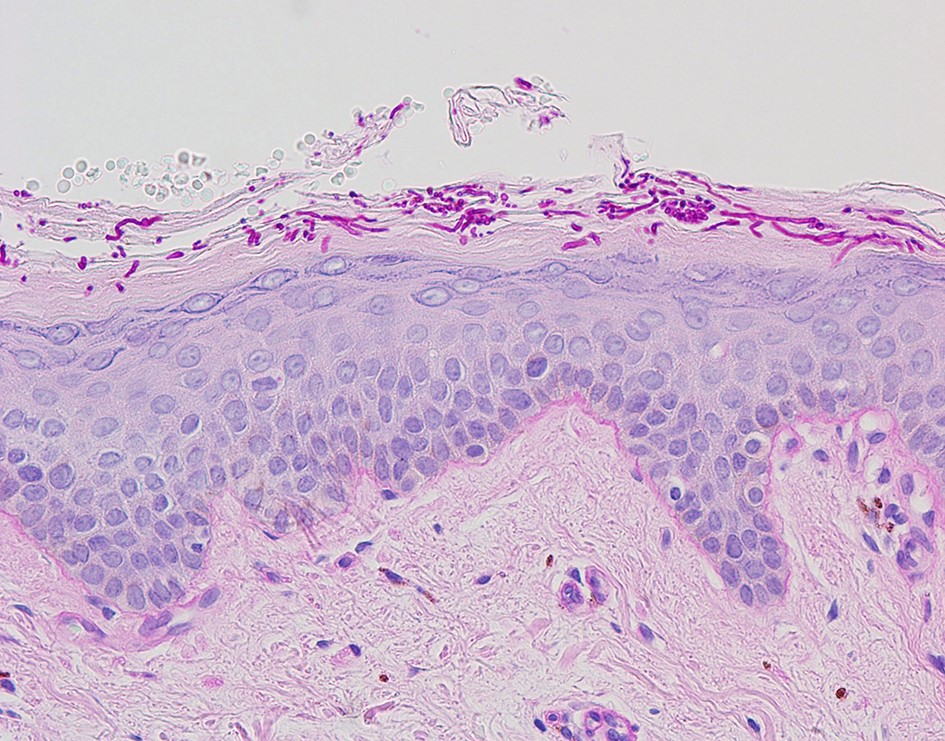
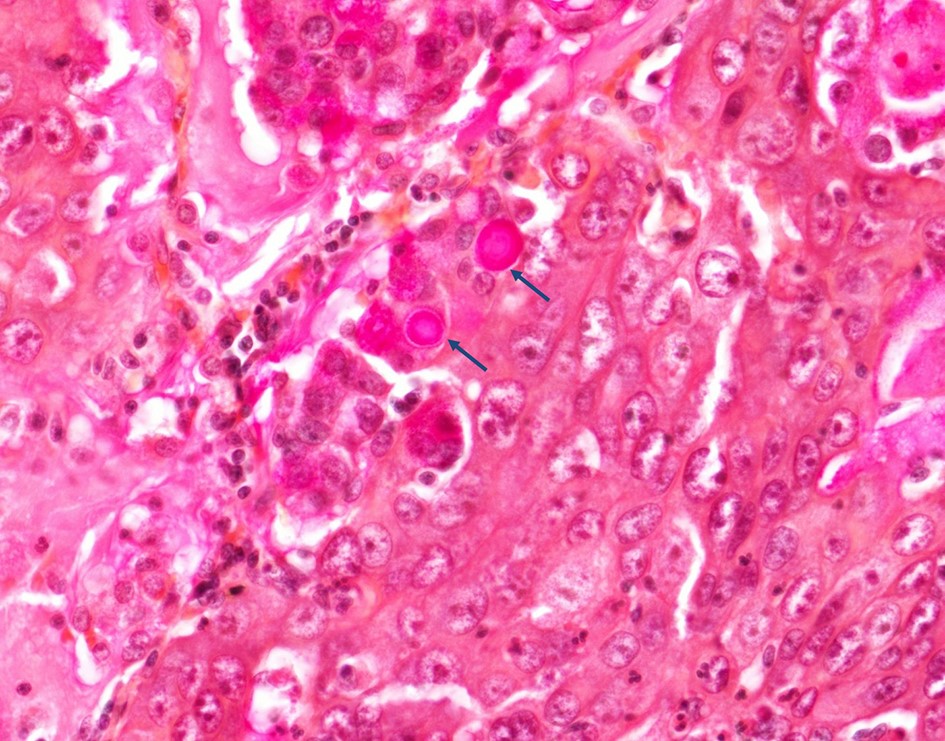
The possibility of both superficial and deep dermatomycoses should be considered in the tissue examination of patients with dermatomycosis. Therefore, the areas to be carefully examined when performing a biopsy are the stratum corneum, tissue around the hair follicles, dermis, and subcutaneous fat layer (Fig. 1). The most basic staining method in skin biopsy is hematoxylin-eosin (H&E) staining, which identifies the presence of fungi, including their hyphae or spores in the stratum corneum, parakeratosis, neutrophil invasion in the epidermis and dermis, purulent folliculitis, follicle destruction, and granuloma1,2,5. In particular, the stratum corneum should be examined first, which are commonly invaded by causative fungi of superficial dermatomycoses; sandwich signs are representative during tissue examination6. In general, if hyphae exist in the stratum corneum, the diagnosis can be confirmed. However, it is often difficult to distinguish hyphae from normal skin structures in H&E staining, so finding a sandwich sign is helpful in diagnosing superficial dermato- mycosis. The sandwich sign is the presence of keratin with dyskeratosis or thick keratinization in the lower part of the stratum corneum and normal keratinization in the upper part. These findings can be seen when fungal hyphae or spores invade the stratum corneum (Fig. 2). Therefore, if a sandwich sign is visible in a biopsy specimen, confirming the presence of hyphae or spores through special staining is necessary to discriminate mycoses (Fig. 3A, 3B). Other clues to fungal infection include compact orthokeratosis without any other explanations and spongiosis, particularly palmo- plantar5. The hyphae in superficial dermatomycosis are often found in the hair follicles7. When identifying fungi invading the hair follicles, it is helpful to observe whether it is ectothrix or endothrix depending on the location of arthroconidia formation15. If spores are located inside the hair shaft, they are classified as endothrix, and if they are located outside, they are classified as ectothrix infections. In ectothrix infection, the cuticle is destroyed, and several species show a yellow-green fluorescence on Wood's lamp test. In endothrix infection, the cuticle is normal and does not emit fluorescence on Wood's lamp test (Table 1)15. In ectothrix infection, Microsporum canis, M. audouinii, M. distortum, M. ferrugineum, M. gypseum, M. nanum, and Trichophyton verrucosum can be suspected as causative fungi. In endothrix infection, T. tonsurans, T. violaceum, and T. soudanense are common causative fungi (Fig. 4). Deep dermatomycosis affects the deeper skin layers below the dermis or beyond the muscle layer or other organs. Since it undergoes a long-term inflammatory reaction, it mainly shows granulomatous changes8. Therefore, it is helpful to closely observe the surrounding tissues infiltrated with inflammatory cells in tissue findings. In superficial der- matomycosis, substances that cannot be found in normal human skin are often a clue. Some fungi form specific vesicles or granules that aid in the diagnosis. For example, in patients with sporotrichosis, asteroid bodies can be observed, and cigar-shaped bodies are rarely seen. In chromoblastomycosis, the causative organism has a characteristic brown color, which are sclerotic bodies or copper pennies (Fig. 5)9. This finding is not observed in brownish phaeohyphomycosis, so it helps to differentiate the two. In addition, sulfur granule is characteristically observed in mycetoma, and the causative fungus can be classified according to the color of sulfur granules, which have high diagnostic value10. In some fungi, their mycelia are branching at a certain angle, so knowing this is helpful in the diagnosis. For example, fungi with branching mycelia at 45° include Aspergillus, Fusarium, and Pseudallescheria boydii. The mycelia of Zygomycetes and Mucorales branch at 90°8 (Fig. 6)9. The angle of hyphae provides necessary insights to make a diagnosis based on H&E stains; the results may be equivocal and may require additional laboratory studies to be confirmed11.
Moreover, while mycotic infections often show close-to-normal pathologic findings, follow-up assessments are warranted if clinical presentations suggest mycotic infections5.
|
|
Ectothrix |
Endothrix |
|
Arthroconidia |
On
the surface of |
Within
the hair shaft |
|
Hyphae |
Within
and out of |
Within
the hair shaft |
|
Hair cuticle |
Destroyed |
Remain
intact |
|
Under Wood's |
Yellow-green
fluorescence +/- |
No
fluorescence |
CHEMICAL STAINING IN DERMATOMYCOSIS
Unlike human cells, fungi have a cell membrane composed of chitin and glycoprotein. Using these characteristics, it is possible to distinguish the causative fungal species by per- forming special staining. H&E staining can also observe hyphae or spores, but it is often difficult to distinguish hyphae from normal skin structures, such as collagen fibers. In addition, if a wrong steroid ointment applied at the beginning of the onset is repeated, the skin would have transformed into an atypical lesion, and it would be easy to misdiagnose the fungal infection12.
In addition to H&E staining, typical chemical staining, such as periodic acid-Schiff (PAS), Gomori's methenamine silver (GMS), Gridley fungus (GF), mucin (alcian blue, mucicarmine), melanin (Fontana Masson), and acid-fast are also available. In each staining method, fungi are stained with a specific color, so that hyphae or spores can be easily identified13.
1. Gomori's methenamine silver stain (GMS stain)
GMS staining, like PAS and GF staining, is mainly used to screen for fungal infections. The cell wall of fungi is stained black, and the surrounding skin tissue becomes dark green (Fig. 7). GMS staining is more sensitive than PAS staining because chromic acid, a stronger oxidizing agent than periodic acid, is used in the staining process. Therefore, degenerated hyphae that are not well-stained with PAS staining are pro- minently observed in GMS staining. On the other hand, if there is a structure that has a dark color, such as melanin, it is difficult to distinguish it from fungi. In addition, it is difficult to observe inflammatory reactions accompanying the fungal infection compared to PAS staining14. Considering these points, both GMS and PAS staining are useful in screening for fungal infections, but there are differences in their usefulness in some cases. For example, for Histoplasma and Aspergillus infections and onychomycosis, GMS staining is more sensitive than PAS staining. For phaeohyphomycosis and Sporothrix, Candida, Blastomyces, Exophiala, Malassezia, and Rhino- sporidium infections, PAS staining is advantageous to observe the lesions (Table 2)5,13.

|
|
GMS stain |
PAS stain |
|
Advantages |
- More
sensitive than PAS |
- Demonstrates fungal morphology better |
|
- Produces
better staining contrast |
||
|
Disadvantages |
|
- Degenerated fungi are refractory to PAS |
|
- Not
adequate for demonstrating inflammatory response |
- Calcific bodies mimic the budding yeasts |
|
|
- Mask
the natural color of pigmented fungi |
- Dead fungi do not always stain |
|
|
|
- The equine fungus Phythium does not stain. |
|
|
Applications |
Superior
in visualizing Histoplasmosis, Aspergillosis, Onychomycosis |
Superior in visualizing |
|
Candidaiasis, Blastomycosis, Phaeohyphomycosis |
2. Periodic acid-Schiff (PAS) stain
In PAS staining, the glycoproteins in the fungal cell wall are stained red, and it is usually used for screening along with GMS and GF staining (Fig. 8). Compared to other staining, PAS staining is suitable for observing the shape of fungi causing the infection and the surrounding skin structure10. Mohan et al.12 said that PAS staining is cheaper, easier, and faster than GMS, which helps in diagnosing dermatomycosis. PAS staining has shortcomings despite its many advantages. It cannot stain phythium and often fails to stain dead fungi, requiring complementary measures, such as GMS staining5.
3. Gridley fungus (GF) stain
GF staining dyes the fungal cell walls purple and the surrounding skin tissues yellow. Like PAS and GMS staining, it is mainly used for screening by staining most of the fungal cell walls.
4. Mucin stain (Alcian blue, mucicarmine)
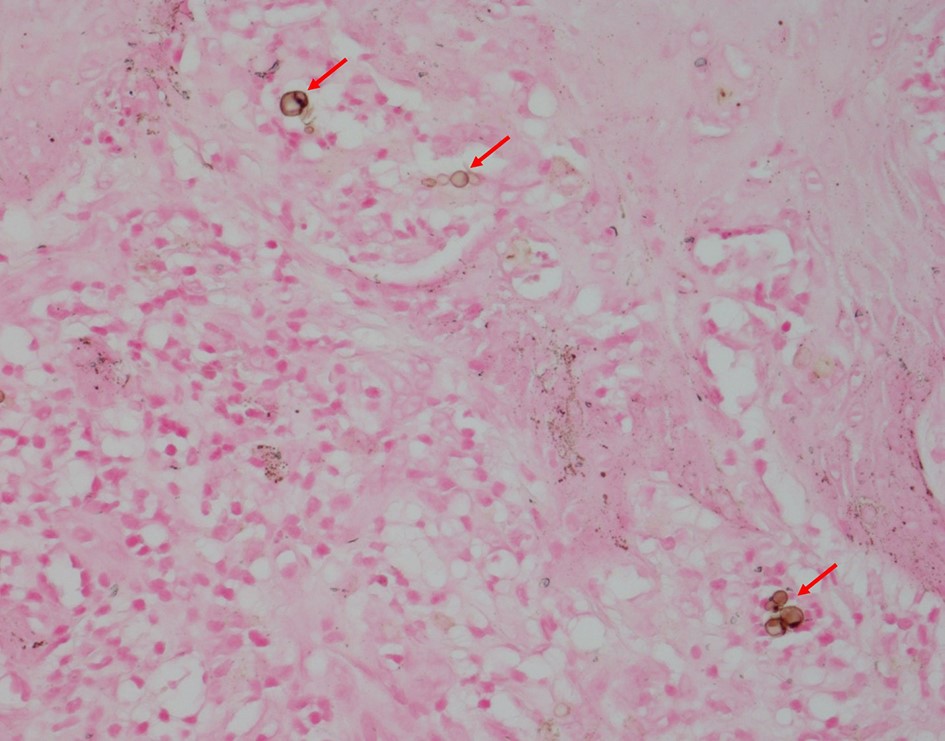
Representative staining include alcian blue and mucicarmine staining, and these are mainly used to diagnose cryptococcosis. When staining with alcian blue, the capsule of Cryptococcus is stained blue, and when stained with mucicarmine, it is stained red (Fig. 9)9.
5. Melanin stain (Fontana Masson)
Fungal cell walls and melanin are stained black. It is mainly useful for diagnosing cryptococcosis, phaeohypomycosis, and chromoblastomycosis (Fig. 5)9.
6. Bacterial stains (Gram stain and acid-fast stain)
Gram stain and acid-fast stain are mainly used for bacterial infections, but specific fungi can also be stained to aid in the diagnosis14. For example, Candida appears blue on Gram stain, while Blastomyces or Histoplasma are stained red by acid-fast stain staining.
Most dermatomycoses in clinical practice are superficial dermatomycosis. This can be easily diagnosed through KOH smear or culture test, so biopsy is not commonly performed. Biopsy is one of the most basic examinations for diagnosis in dermatology, including the diagnosis of dermatomycosis. The positive rate of biopsy is higher than that of other tests, and its diagnostic value is particularly high in deep dermatomycosis or onychomycosis. In addition, if the results of KOH smear or culture test are negative in patients with superficial dermatomycosis, it is easy to overlook the possibility of dermatomycosis. Therefore, it can be misdiagnosed as other diseases, such as skin tuberculosis, skin bacterial infection, and skin tumors. Thus, it is important to consider dermatomycosis when examining a patient in a clinical setting. If a skin lesion that has a chronic course or a nonspecific skin lesion that recurs repeatedly is observed in a patient with reduced immune function, skin biopsy must be performed to confirm the presence of skin mycosis.
Moreover, since the causative fungi can be identified through appropriate special staining, it is important to keep in mind the kind of staining that is suitable for each fungus.
References
1. Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, et al. Fitzpatrick's dermatology. 9th ed. New York: McGraw-Hill, 2019:2925-2988
2. KDA Textbook Editing Board. Dermatology. 7th ed. Seoul: McGraw-Hill, 2020:332-354
3. Kim TK, Jeon YS, Kim ST, Suh KS. The clinical, histo- pathologic and mycologic characteristics of dermato- phytosis which were initially diagnosed by skin biopsies. Korean J Dermatol 2009;47:1345-1352
Google Scholar
4. Cho BK. Diagnoses and differential diagnoses of super- ficial mycoses. Korean J Med Mycol 2001;6:49-56
Google Scholar
5. Patterson JW. Weedon's skin pathology. 5th ed. China: Elsevier, 2021:31-45
6. KDA Textbook Editing Board. Color atlas of dermato- pathology. 3nd ed. Seoul: Daehan medicine, 2017:293-310
7. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/ MSG) Consensus Group. Clin Infect Dis 2008;46:1813-1821
Google Scholar
8. Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Fungal disease, In: Lever's histopathology of the skin. 11th ed. Philadelphia: Wolters Kluwer, 2015:727-760
9. Park JS, Kim HR. Histopathology and Histochemistry in Cutaneous Fungal Infections. Korean J Med Mycol 2016; 21:1-7
Google Scholar
10. Calonje E, Brenn T, Lazar AJ, Billings SD. Infectious dis- ease of the skin, In: Mckee's Pathology of the Skin. 5th ed. China: Elsevier, 2020:826-975
11. Choi SI, Song JS, Kim JY, Cha HH, Yun JH, Park JW, et al. Diagnostic performance of immunohistochemistry for the aspergillosis and mucormycosis. Mycoses 2019;62: 1006-1014
Google Scholar
12. Liber AF, Choi HS. Splendore-Hoeppli phenomenon about silk sutures in tissue. Arch Pathol 1973;95:217-220
Google Scholar
13. Mohan H, Bal A, Aulakh R. Evaluation of skin biopsies for fungal infections: role of routine fungal staining. J Cutan Pathol 2008;35:1097-1099
Google Scholar
14. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev 2011; 24:247-280
Google Scholar
15. Rippon JW. Medical mycology 3rd ed. Philadelphia: WB Saunders, 1998:186-194
Congratulatory MessageClick here!