pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Ji-In Seo ,Min-Kyung Shin
10.17966/JMI.2020.25.4.65 Epub 2021 January 05
Abstract
Onychomycosis is a fungal infection of the nail unit. Compared to other superficial dermatophytoses of the skin and hair, onychomycosis is associated with a chronic course and frequent recurrence. Originally, structural characteristics of the nail, such as poor vascular supply and thick layers of hard keratin, were thought to be limiting factors for efficient drug delivery, resulting in prolonged treatment. However, recent research reveals the following crucial mechanisms contributing to the chronicity of nail dermatophytosis: innate characteristics of Trichophyton rubrum, formation of dormant spores called arthroconidia, physical and immunologic characteristics of the nail apparatus, and alteration of the defense system of the host. T. rubrum, the most common causative fungal pathogen for onychomycosis, acquires selective immune tolerance toward the host's defense system. Transformation into dormant phases, such as arthroconidia, creates greater resistance to antifungal medication and nonnutritive environments. The anatomical and biological structures of the nail allow immune evasion, increase infection susceptibility, and promote biofilm formation. Key regulators of the host's innate and adaptive immune systems are downregulated as infection persists, and age-associated immunomodulation aggravates the process.
Keywords
Arthroconidia Dermatophytoma Nail dermatophytosis Onychomycosis
Dermatophyte, nondermatophytic mold, or yeast infection of the nail, termed onychomycosis (OM), is one of the most common superficial fungal infections. Among its causative organisms, Trichophyton rubrum is the most prevalent. Com- pared to other superficial dermatophytoses of the skin and hair, OM is associated with a chronic course and frequent recurrence. As a result, a minimum of three months of systemic antifungal treatment is necessary until complete nail replacement is achieved. The systemic treatment of choice for OM is terbinafine, the most common fungicidal drug used in various dermatophytoses. Unfortunately, only 38% of patients achieve complete remission. Moreover, recurrence—including relapse and reinfection—occur in approximately 6.5~53% of cases
Initially, the structural characteristics of the nail, such as poor vascular supply and three thick layers of hard keratin—the dorsal, intermediate, and ventral layers—were thought to be limiting factors for efficient drug delivery, resulting in prolonged treatment. However, recent research has revealed the following four major mechanisms contributing to the chronicity of OM: innate characteristics of T. rubrum, formation of arthroconidia, physical and immunologic characteristics of the nail unit, and alteration of defense system of the host.
T. rubrum is the causative pathogen in 71% of all dermato- phytoses and responsible for 90% of chronic dermatophytosis cases. Over time, the pathogen has developed several adaptive features to evade the immune surveillance of its human host. Among them, the ability to escape identification by pattern recognizing receptors (PRPs) of the host's immune system through pathogen-associated molecular patterns (PAMPs) has been the most effective. Specific molecules or proteins produced by the fungus that hinder the antigen recognition process of PAMPs have been identified. The polysaccharide mannan in the cell wall of T. rubrum, for ex- ample, inhibits critical steps in the antigen-presenting process. Furthermore, the lipophilic toxin associated with mannan has been shown to inhibit the cellular immunity of the host. Similarly, Muraosa et al. identified certain proteins induced by chitin—a common component of fungal cell walls—inhibiting the immune responses of the host. Specifically, the study found Lysin motif (LysM)-domains A (LdpA) and B (LdpB) had decreased catalytic function on chitin, despite normal chitin recognition ability. This decrease in chitin catalysis greatly benefits fungal survival. In summary, dermatophytes acquire selective immune tolerance as the infection becomes chronic, in addition to their innate immune evasion abilities.
Arthroconidia are spores formed by the segmentation and fragmentation of pre-existing hyphae. Spores remain in the stratum corneum and nail, evading the immune surveillance of the host. Under favorable conditions, arthroconidia trans- form back into actively propagating hyphae and invade the viable epidermis. Despite the nail bed lacking nutritional sources, arthroconidia is resistant to unfavorable environmental conditions and may survive inside the nail for long periods. Hence, the dormant spore is considered the primary form for nail fungal invasion, acting as a reservoir during reinfection and relapse.
Various factors are known to induce arthroconidium produc- tion. The reaction of host tissue to fungal hyphal growth (e.g., subungual hyperkeratosis) acts as an environmental change and promotes spore formation9. Also, sub-inhibitory levels of antifungal medications and use of betamethasone stimu- late arthroconidia formation. Consequently, it is crucial for fungicidal drugs to attain sufficient concentrations in order to eliminate fungal spores and prevent recurrence.
However, merely aiming to exceed the minimum fungicidal concentration (MFC) of terbinafine against T. rubrum may be inadequate as shown by standard laboratory assays. Since most antifungal agents target the hyphae's growth stage, drug potency dramatically decreases in the arthroconidia phase. Eradication of T. mentagrophytes in the arthroconidia phase, for instance, requires over 1,000-fold terbinafine concen- tration than that in the hyphae growth phase. Similarly, the potency of terbinafine against Microsporum species decreases by more than half in the conidia phase. Subsequently, arthroconidia is hypothesized as the main reason for the 100-fold MFC increase seen in human nail powder compared to that in standard media. In summary, dormant spores highly contribute to treatment resistance observed in OM, and their eradication is of paramount importance in preventing recurrence.
Although the permeability per unit thickness is much greater than that of the stratum corneum, the thick nail plate acts as a physical barrier to drug delivery. Such physical char- acteristics impede the penetration of systemic and topical drugs, resulting in OM treatment resistance. Consequently, concomitant systemic (inside approach) and topical (outside approach) treatment have been recommended in order to attain sufficient antifungal drug concentrations. Yet, in addition to hindering drug delivery, the structural durability of the nail reinforces immune evasion, infection susceptibility, and dermatophytoma formation.
The anatomy of the nail allows fungal pathogens to evade the immune surveillance of the host. Contact with antigen-presenting cells are limited since fungal pathogens remain intact within keratin fibers. Additionally, certain areas of the nail present with different compositions of immune cells and immunomodulators. Immunohistological studies show that the proximal nail matrix particularly possesses elevated levels of immunosuppressants, including transforming growth factor beta 1, alpha melanocyte-stimulating hormone, and insulin-like growth factor 1, while few immune cells, such as cluster of differentiation (CD) 1a, CD4, CD8, natural killer, and mast cells. In other words, certain locations of the nail unit are associated with relatively low immune capacity. The anti-inflammatory milieu of the nail unit inhibits the appro- priate defense system and results in persistent dermatophyte infection.
Secondly, the slow growth rate of the nail increases sus- ceptibility to infection and prolongs treatment course. Invasion of fungal pathogens involves adherence of the arthroconidia to keratinized tissue and germination through hyphal pro- duction. In order to achieve continuous hyphal growth, the speed of spore germination must be faster than the nail's growth rate. Thus, the slow growth rate of the nail provides abundant time for fungal mycelial growth and increases susceptibility to dermatophyte infection. The process is further augmented in OM, since individuals with nail dermatophytosis have slower nail growth rates than the healthy population. However, whether the slow growth rate is a result of the dermatophyte infection or host conditions—such as old age, comorbidities, or both—remains undetermined.
Lastly, the physical properties of the nail contribute to bio- film formation. Although fungi are generally known to be planktonic organisms, they form a special habitat called bio- film. Inside, fungal pathogens are aggregated and surrounded by an extracellular polymer matrix. The rigid outer structure functions as a barrier against the host's defense system and antifungal drugs. Moreover, the densely assembled fungal pathogens can increase virulence and gain multiple drug resistance by quorum sensing (QS). QS is a "density-dependent signaling mechanism", in which the accumulation of small signaling molecules secreted by fungal organisms controls gene expression in a community-based manner. Most of the causative fungal agents of dermatophytosis, such as T. rubrum, T. mentagrophytes, Candida, Aspergillus, and Fusarium, form biofilms. In a study conducted by Mustafa et al. 28 out of 54 cultured samples (51.8%) from OM patients demonstrated biofilm formation. Biofilm structures called spikes, strikes, or fungal balls are visible to the naked eye as dermatophytomas. Hence, in addition to antifungal medication, physical removal of visible dermato- phytomas is recommended. Aside from the physical char- acteristics of the nail, diabetes mellitus, repetitive minor nail trauma, nail humidity, and failed prolonged systemic anti- fungal treatment are also predisposing factors for biofilm formation.
Mechanisms related to defects in innate and adaptive immunity due to OM are still not clearly understood. Adaptive immunity has been the central player in the defense against opportunistic infections. The delicate equilibrium between T helper (Th)-1/Th17 cells is important in maintaining antifungal resistance. On the contrary, switching to the Th2 response permits pathogen persistence and immune tolerance through immunological memory. Regarding innate immunity, impaired phagocytosis and reduced production of free radicals or nitric oxide are seen in various fungal infections, including T. rubrum. Defects in innate and adaptive immunity simultaneously affect each other and aggravates the process. T. rubrum infection downregulates the toll-like receptor (TLR) 4 pathway, which in turn suppresses pro-inflammatory cyto- kines such as tumor necrosis factor-α and interleukin (IL)-1β25. With the combination of defective phagocytosis and insufficient immune-related inflammatory response, the risk of chronic dermatophytosis increases.
Many patients with OM are unable to elicit a cell-mediated immune response, as demonstrated by the negative or significantly reduced trichophytin antigen intradermal skin test results. Several hypotheses have been proposed to explain the lack of cellular immunity despite persistent and recurrent infections. First, neonatal exposure to the fungus or cross-reacting antigens to microbial pathogens may induce immune tolerance. Second, the activation of specific sup- pressor T cells causes persistent infection. Lastly, incomplete antigen penetration through the stratum corneum hinders the induction of anti-defense immunity. This insufficient immune response to T. rubrum is notably observed in patients with chronic dermatophytosis, mostly in those infected over five years. Hence, prolonged infection increases chances of selective immune tolerance.
Immunosenescence, which is defined as an age-associated modification of the immune system, is one of the major risk factors for OM. Considering how the prevalence of nail dermatophytosis is near 20% in people aged over 60 years, but less than 5.5% in children and infants31, such notion is predictable. In regards to the adaptive immune system, immunosenescence induces the predominance of the Th2 response and promotes chronic fungal colonization. In- creased Th2/Th1 ratio expands IL-4, IL-6, and IL-10 cytokine profiles, leading to impaired cell-mediated immunity and phagocytosis. Concurrently, circulation of IL-17 and senes- cent dendritic cells decreases immunity, favoring commen-salism of the host and fungus. Concerning innate immunity, the functions of PRPs and recruitment of phagocytes decline. Given that fungal cell wall components—chitin, mannan, β-glucan, etc.—are main sources of PAMPs, ineffective PRPs allow immune tolerance. Among them, decreased TLR 4 expression has been noted in several fungal diseases to cause suppression of pro-inflammatory cytokines25. Additionally, age-related TLR impairment may result from an increase in advanced glycation end products, which inhibit the biological activity of the receptor.
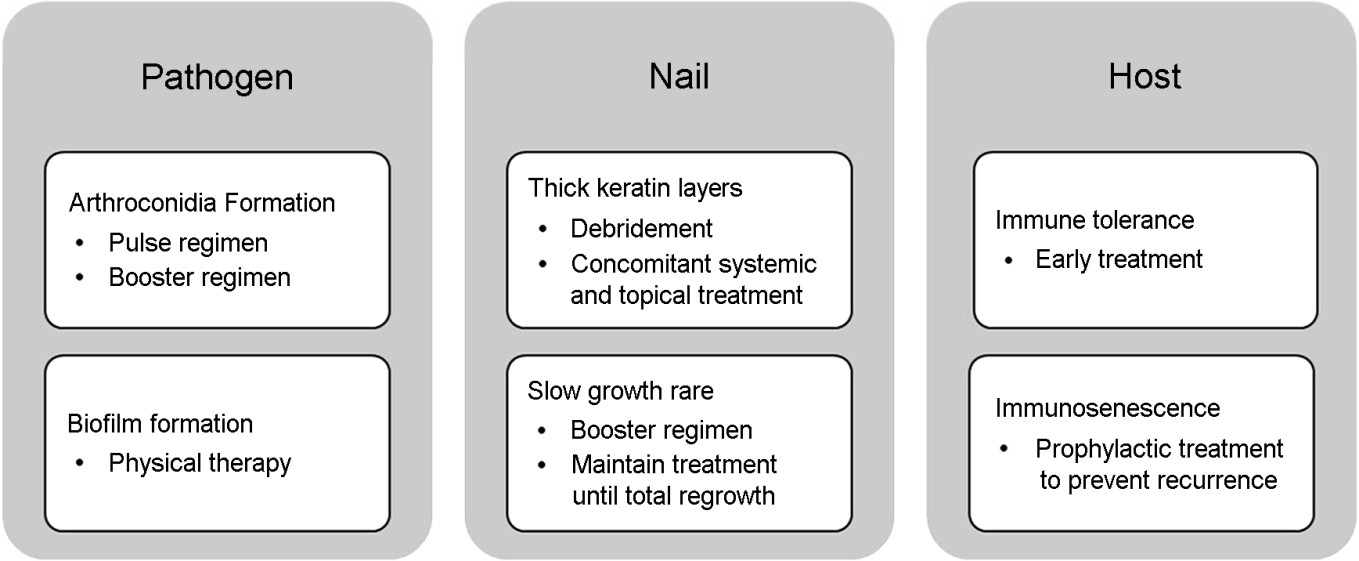
Among superficial fungal infections, OM is the most resistant to treatment. Despite advancements in treatment over the past decades, chronic and recurrent nail dermato- phytosis persists. Recent research suggests that the combination of innate traits of T. rubrum, existence of arthroconidia, structural and immunological features of the nail unit, and alteration of the defense system of the host contribute to the chronicity of onychomycosis.
Maintaining adequate concentrations of the antifungal drug is important for overcoming immune evasion. Since subminimum inhibitory concentrations promote biofilm and arthroconidia formation, exceeding the minimum inhibitory concentration is crucial for complete cure. To reach high concentrations in the nail itself, concomitant treatment with systemic and topical antifungal agents is recommended. In controlled trials, combined treatment of oral terbinafine and 5% topical amorolfine significantly increases treatment success rates compared to oral terbinafine alone. If consistent long-term treatment is impossible, an additional booster treatment 6 to 9 months after the standard systemic anti- fungal therapy is recommended. To maximize arthroconidia eradication, pulse regimen is recommended over continuous. In pulse regimens, the drug-free weeks allow transformation of dormant conidia to hyphae growth forms, leading to greater drug potency. Although the mycological cure rate is higher in the continuous regimen than that in the pulse regimen, meta-analyses show both regimens are equally effective in achieving complete cure. In addition to dosage, the timing of treatment is critical for increasing the complete response success rate. Chronic infections lasting over five years have a higher chance of inducing selective immune tolerance. Therefore, dermatologists should encourage early treatment upon diagnosis. Moreover, for patients with a family history of immune deficiency or fungal disease susceptibility, aggressive treatment should be initiated prior to OM progression. The causes of recalcitrant OM and cor- responding treatment strategies are summarized in Fig. 1.
Novel approaches for future treatment are currently under investigation. Fungal quorum sensing inhibitor (QSI), for ex- ample, reduces gene expression regulated by QS. As the density of fungal pathogens increases, QSIs accumulate in the extracellular environment, eventually inhibiting mycelial growth and biofilm formation. One example is the farnesol, which is secreted by Candida albicans during sterol synthesis16. Farnesol represses hyphal filamentation by downregulating the adenylyl cyclase pathway, ultimately inhibiting Cyr1 and Nrg1. Therefore, QSIs may be a candidate therapy for inhi- biting fungal biofilm formation. In conjunction with antifungal agents, laser and light therapy are attempted as adjuvant modalities. Lasers, such as 1,064 nm neodymium-doped yttrium aluminum garnet (Nd:YAG) and 420 nm intense pulsed light, reduce the biofilm viability of C. albicans by 45~60%. Likewise, photodynamic therapy demonstrates potential activity against biofilm formation in C. albicans.
For advancements in the medical management of OM, understanding the principal mechanisms of treatment re- sistance is of foremost importance. Long-term antifungal therapy, concomitant systemic and topical treatment, and biofilm destructing adjuvant laser treatments have increased cure rates over time. However, owing to the increasingly aging population and increased use of occlusive footwear during leisure activities, the prevalence of OM continues to rise and poses a serious public health problem. Therefore, continuous research on the ecological system of fungal pathogens and the pharmacokinetics of antifungal agents is crucial for future OM management.
References
1. Bodman MA, Krishnamurthy K. Onychomycosis. In: Stat- Pearls. Treasure Island (FL): StatPearls Publishing LLC; August 2020
2. Tosti A, Elewski BE. Onychomycosis: Practical approaches to minimize relapse and recurrence. Skin Appendage Disord 2016;2:83-87
Google Scholar
3. Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses 1989;32:609-619
Google Scholar
4. Hay RJ. Chronic dermatophyte infections. I. clinical and mycological features. Br J Dermatol 1982;106:1-7
Google Scholar
5. Zheng NX, Wang Y, Hu DD, Yan L, Jiang YY. The role of pattern recognition receptors in the innate recognition of Candida albicans. Virulence 2015;6:347-361
Google Scholar
6. Muraosa Y, Toyotome T, Yahiro M, Kamei K. Character- isation of novel-cell-wall LysM-domain proteins LdpA and LdpB from the human pathogenic fungus Aspergillus fumigatus. Sci Rep 2019;9:3345-019-40039-1
Google Scholar
7. Blake JS, Dahl MV, Herron MJ, Nelson RD. An immuno- inhibitory cell wall glycoprotein (mannan) from Tricho- phyton rubrum. J Invest Dermatol 1991;96:657-661
Google Scholar
8. Dahl MV. Dermatophytosis and the immune response. J Am Acad Dermatol 1994;31:S34-41
Google Scholar
9. Yazdanparast SA, Barton RC. Arthroconidia production in Trichophyton rubrum and a new ex vivo model of onychomycosis. J Med Microbiol 2006;55:1577-1581
Google Scholar
10. Seebacher C. Action mechanisms of modern antifungal agents and resulting problems in the management of onychomycosis. Mycoses 2003;46:506-510
Google Scholar
11. Fernandez-Torres B, Inza I, Guarro J. Comparison of in vitro antifungal susceptibilities of conidia and hyphae of dermatophytes with thick-wall macroconidia. Antimicrob Agents Chemother 2003;47:3371-3372
Google Scholar
12. Osborne CS, Leitner I, Favre B, Ryder NS. Antifungal drug response in an in vitro model of dermatophyte nail infection. Med Mycol 2004;42:159-163
Google Scholar
13. Ito T, Ito N, Saathoff M, Stampachiacchiere B, Bettermann A, Bulfone-Paus S, et al. Immunology of the human nail apparatus: The nail matrix is a site of relative immune privilege. J Invest Dermatol 2005;125:1139-1148
Google Scholar
14. Geyer AS, Onumah N, Uyttendaele H, Scher RK. Modula- tion of linear nail growth to treat diseases of the nail. J Am Acad Dermatol 2004;50:229-234
Google Scholar
15. Gupta AK, Daigle D, Carviel JL. The role of biofilms in onychomycosis. J Am Acad Dermatol 2016;74:1241-1246
Google Scholar
16. Albuquerque P, Casadevall A. Quorum sensing in fungi-a review. Med Mycol 2012;50:337-345
Google Scholar
17. Padder SA, Prasad R, Shah AH. Quorum sensing: A less known mode of communication among fungi. Microbiol Res 2018;210:51-58
Google Scholar
18. Favre-Godal Q, Gourguillon L, Lordel-Madeleine S, Gindro K, Choisy P. Orchids and their mycorrhizal fungi: An in- sufficiently explored relationship. Mycorrhiza 2020;30: 5-22
Google Scholar
19. Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Crit Rev Microbiol 2009;35:340-355
Google Scholar
20. Costa-Orlandi CB, Sardi JC, Santos CT, Fusco-Almeida AM, Mendes-Giannini MJ. In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling 2014;30:719-727
Google Scholar
21. Imamura Y, Chandra J, Mukherjee PK, Lattif AA, Szczotka-Flynn LB, Pearlman E, et al. Fusarium and Candida albicans biofilms on soft contact lenses: Model development, in- fluence of lens type, and susceptibility to lens care solutions. Antimicrob Agents Chemother 2008;52:171-182
Google Scholar
22. Mustafa MR, El-Samahy MH, Diab HM, Bendary SE. Clinico-mycological study of fungal biofilms in recalcitrant onychomycosis. Mycobac Dis 2017;7:234
23. Sparber F, LeibundGut-Landmann S. Interleukin-17 in antifungal immunity. Pathogens 2019;8:54
Google Scholar
24. van de Veerdonk FL, Netea MG. T-cell subsets and anti- fungal host defenses. Curr Fungal Infect Rep 2010;4: 238-243
Google Scholar
25. Oliveira CB, Vasconcellos C, Sakai-Valente NY, Sotto MN, Luiz FG, Belda Junior W, et al. Toll-like receptors (TLR) 2 and 4 expression of keratinocytes from patients with localized and disseminated dermatophytosis. Rev Inst Med Trop Sao Paulo 2015;57:57-61
Google Scholar
26. de Sousa Mda G, Santana GB, Criado PR, Benard G. Chronic widespread dermatophytosis due to Trichophyton rubrum: A syndrome associated with a Trichophyton-specific functional defect of phagocytes. Front Microbiol 2015;6:801
Google Scholar
27. Roeder A, Kirschning CJ, Rupec RA, Schaller M, Weindl G, Korting HC. Toll-like receptors as key mediators in in- nate antifungal immunity. Med Mycol 2004;42:485-498
Google Scholar
28. Elewski BE, El Charif M, Cooper KD, Ghannoum M, Birnbaum JE. Reactivity to trichophytin antigen in patients with onychomycosis: Effect of terbinafine. J Am Acad Dermatol 2002;46:371-375
Google Scholar
29. Hay RJ, Brostoff J. Immune responses in patients with chronic Trichophyton rubrum infections. Clin Exp Dermatol 1977;2:373-380
Google Scholar
30. Turk JL. An experimental model for the investigation of the cellular basis of desensitization in contact sensitivity. Int Arch Allergy Appl Immunol 1965;28:105-112
Google Scholar
31. Gupta AK, Versteeg SG, Shear NH. Onychomycosis in the 21st century: An update on diagnosis, epidemiology, and treatment. J Cutan Med Surg 2017;21:525-539
Google Scholar
32. Aw D, Silva AB, Palmer DB. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007; 120:435-446
Google Scholar
33. Alberti S, Cevenini E, Ostan R, Capri M, Salvioli S, Bucci L, et al. Age-dependent modifications of type 1 and type 2 cytokines within virgin and memory CD4+ T cells in humans. Mech Ageing Dev 2006;127:560-566
Google Scholar
34. Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 2012;24:331-341
Google Scholar
35. Chen YJ, Sheu ML, Tsai KS, Yang RS, Liu SH. Advanced glycation end products induce peroxisome proliferator-activated receptor gamma down-regulation-related in- flammatory signals in human chondrocytes via toll-like receptor-4 and receptor for advanced glycation end pro- ducts. PLoS One 2013;8:e66611
Google Scholar
36. Baran R, Sigurgeirsson B, de Berker D, Kaufmann R, Lecha M, Faergemann J, et al. A multicentre, randomized, con- trolled study of the efficacy, safety and cost-effectiveness of a combination therapy with amorolfine nail lacquer and oral terbinafine compared with oral terbinafine alone for the treatment of onychomycosis with matrix involvement. Br J Dermatol 2007;157:149-157
Google Scholar
37. Gupta AK, Baran R, Summerbell R. Onychomycosis: Strategies to improve efficacy and reduce recurrence. J Eur Acad Dermatol Venereol 2002;16:579-586
Google Scholar
38. Gupta AK, Paquet M, Simpson F, Tavakkol A. Terbinafine in the treatment of dermatophyte toenail onychomycosis: A meta-analysis of efficacy for continuous and intermittent regimens. J Eur Acad Dermatol Venereol 2013;27:267 -272.
Google Scholar
39. Hall RA, Turner KJ, Chaloupka J, Cottier F, De Sordi L, Sanglard D, et al. The quorum-sensing molecules farnesol /homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans. Eukaryot Cell 2011;10:1034-1042
Google Scholar
40. Vila TV, Rozental S, de Sa Guimaraes CM. A new model of in vitro fungal biofilms formed on human nail frag- ments allows reliable testing of laser and light therapies against onychomycosis. Lasers Med Sci 2015;30:1031 -1039.
Google Scholar
41. Junqueira JC, Jorge AO, Barbosa JO, Rossoni RD, Vilela SF, Costa A, et al. Photodynamic inactivation of biofilms formed by Candida spp., Trichosporon mucoides, and Kodamaea ohmeri by cationic nanoemulsion of zinc 2,9,16,23-tetrakis(phenylthio)-29H, 31H-phthalocyanine (ZnPc). Lasers Med Sci 2012;27:1205-1212
Google Scholar
Congratulatory MessageClick here!