pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Jong Heon Jeong,Ji Young Lee,Seung Ho Lee, Ai-Young Lee ,Jong Soo Hong
10.17966/JMI.2020.25.3.51 Epub 2020 October 06
Abstract
Background: The kinds and identification rates of major bacteria causing bacterial skin infections have changed steadily over time. Although studies of the causative pathogen changes over time have been reported worldwide, no follow-up studies of bacterial skin infections have been conducted in Korea since 2009.
Objective: To investigate the changes in the identification rate and antibiotic susceptibility of causative pathogens of bacterial skin infections, especially S. aureus, over time in Korea.
Methods: This study was conducted in outpatient clinics in 2006, 2009, 2012, 2015, and 2018, in which the data on age, sex, identification of bacteria, and susceptibility to antibiotics were retrospectively analyzed.
Results: Of the 350 cases, except for suspected normal flora, 213 (60.9%) were positive for S. aureus, accounting for the highest percentage, followed by 22 (6.3%) for P. aeruginosa, 7 (2%) for Serratia marcescens, and 6 (1.7%) for Bacillus species, Enterococcus faecalis, and Staphylococcus lugdunensis. The identification rate of methicillin-resistant S. aureus (MRSA) increased from 2009 to 2015 and then decreased in 2018.
Conclusion: The proportion of MRSA gradually increased from 2006 to 2015 but suddenly reversed in 2018, possibly due to improved education for infection prevention or changes in the virulence of the circulating strains of MRSA. Also, based on our findings, MRSA is currently susceptible to trimethoprim-sulfamethoxazole and vancomycin.
Keywords
Antibiotics Bacterial skin infections MRSA S. aureus
Bacterial skin infections can be divided into primary infec- tions (pyoderma) caused by bacterial invasion on normal skin and secondary infections occurring on existing damaged skin. In cases of primary infections, a single type of bacteria is identified in the bacterial cultures, whereas in cases of sec- ondary infections, more than one bacterium can be mixed in the cultures. Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), and Streptococcus pyogenes (S. pyogenes) are common pathogens of bacterial skin infections in bacterial cultures, the kinds, and identification rates of which have changed steadily over time. Because strains have become penicillin-resistant, antibiotics, such as cephalosporin, methicillin, and oxacillin, have been developed. However, methicillin-resistant S. aureus (MRSA), which is resistant to the aforementioned antibiotics, has appeared, along with vancomycin-resistant S. aureus (VRSA)1. Although studies on the changes in causative pathogens over time have been reported worldwide, no follow-up studies on bacterial skin infections have been conducted in Korea since 20092. There- fore, we conducted this study to investigate the changes in the identification rate and antibiotic susceptibility of causative pathogens of bacterial skin infections, especially S. aureus, over time in Korea.
|
Diagnosis |
No. of patients (%) |
Total |
||||
|
2006 |
2009 |
2012 |
2015 |
2018 |
||
|
Secondary
pyoderma |
6
(75) |
49
(83.1) |
100
(70.1) |
187
(80.6) |
120
(75) |
462
(78.3) |
|
Furuncle,
carbuncle |
1
(12.5) |
3
(5.1) |
20
(14.2) |
3
(1.3) |
8
(5.3) |
35
(5.9) |
|
Cellulitis |
1
(12.5) |
0 |
6
(4.3) |
19
(8.2) |
5
(3.3) |
31 (5.3) |
|
Impetigo |
0 |
1
(1.7) |
5
(3.5) |
17
(7.3) |
9 (6) |
32
(5.4) |
|
Paronychia |
0 |
3
(5.1) |
4
(2.8) |
4
(1.7) |
2
(1.3) |
13
(2.2) |
|
Folliculitis |
0 |
3
(5.1) |
6 (4.3) |
2
(0.9) |
6 (4) |
17
(2.9) |
|
Total |
8
(100) |
59 (100) |
141 (100) |
232 (100) |
150 (100) |
590 (100) |
|
|
||||||
We conducted this study at the outpatient dermatology clinics of Dongguk University Ilsan Hospital (DUIH) in 2006, 2009, 2012, 2015, and 2018. We collected the data for skin bacterial cultures from inflammatory, purulent, ulcerous, and exudative lesions suspected to be bacterial infections. Using the patients' electronic medical records, data on age, sex, bacterial identification, and susceptibility to antibiotics, were retrospectively analyzed, and the diagnoses, including sec- ondary pyoderma, furuncles, carbuncles, cellulitis, folliculitis, impetigo, and paronychia, and the antibiotic susceptibility test results were collected. Coagulase-negative staphylococci and normal cutaneous microbiota identified were excluded from the analysis because they can be detected easily in normal healthy skin. This study was approved by the Institutional Review Board of DUIH (DUIH 2019-10-001).
1. Diagnosis of skin infections
In all the years of the study, most of the diagnoses were secondary pyoderma, which is secondary to an underlying skin disease, accounting for 70~80%, followed by furuncle, impetigo, cellulitis, folliculitis, and paronychia. Overall, the incidence of each skin infection did not change significantly every year (Table 1).
2. Bacteriological identification of skin cultures
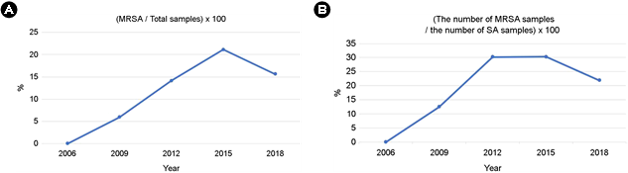
Of the 590 cases that had bacterial cultures for skin lesions, 438 were positive for bacteria, accounting for 74.2% of the total. In 2006, 8 cases that had bacterial skin cultures were all positive for the organism (100%), followed by 43 of 59 (72.9%) in 2009, 111 of 141 (78.7%) in 2012, 165 of 232 (71.1%) in 2015, and 111 of 150 (74%) in 2018. Of the 350 cases, except for suspected normal flora (coagulase-negative staphylococci, normal cutaneous microbiota), 213 cases (60.9%) were positive for S. aureus, accounting for the highest percentage, followed by 22 (6.3%) with P. aeruginosa. When the organisms identified each year were analyzed, S. aureus was the most common pathogen, followed by P. aeruginosa (Fig. 1A, B, Table 2).
3. Antibiotic susceptibility results of S. aureus
The antimicrobial susceptibility tests for S. aureus in the bacterial cultures showed that 90.6% of the isolates were penicillin-resistant, the highest resistance seen. The resistance rates for penicillin were 100% in 2006 and 2009, 95.3% in 2012, 92.1% in 2015, and 82.8% in 2018, whereas the resistance rates for the β-lactam antibiotic oxacillin were 0%, 12.5%, 30.2%, 30.3%, and 21.9% in 2006, 2009, 2015, and 2018, respectively, which increased until 2015 and then decreased in 2018. Other antibiotics, clindamycin, erythro- mycin, and gentamicin, showed significant resistance rates of 20~30%. Only one case was resistant to linezolid and trimethoprim-sulfamethoxazole (TMP-SMX) in 2015 and 2018. The cases were non-resistant to vancomycin (Table 3).
|
Pathogens |
No. of patients (%) |
||||
|
2006 |
2009 |
2012 |
2015 |
2018 |
|
|
S. aureus (MSSA) |
1
(16.7) |
14
(41.2) |
30
(32.6) |
62
(48.4) |
50
(55.6) |
|
MRSA |
0
(0) |
2
(5.9) |
13
(14.1) |
27
(21.1) |
14
(15.6) |
|
S. pyogenes |
0
(0) |
2
(5.9) |
0
(0) |
1 (0.8) |
1
(1.1) |
|
P. aeruginosa |
1
(16.7) |
6
(17.6) |
6
(6.5) |
7
(5.5) |
2
(2.2) |
|
K. pneumoniae |
0
(0) |
0
(0) |
2
(2.2) |
3
(2.3) |
0
(0) |
|
E. coli |
0
(0) |
0
(0) |
1
(1.1) |
0 (0) |
1
(1.1) |
|
Others |
4
(66.7) |
10
(29.4) |
40
(43.5) |
28
(21.9) |
22
(24.4) |
|
Total |
6 (100) |
34 (100) |
92 (100) |
128 (100) |
90 (100) |
4. Frequency of MRSA resistant to β-lactam antibiotics
No cases of MRSA that was also resistant to oxacillin were observed in 2006, but 2 were observed in 2009, 13 in 2012, 27 in 2015, and 14 in 2018 (Table 2). The ratio of MRSA to the total number of specimens suspected of containing pathogens was 0% in 2006, 5.9% in 2009, 14.1% in 2012, 21.1% in 2015, and 15.6% in 2018 (Fig. 1A). The identification rate of MRSA increased from 2009 to 2015 and then decreased in 2018.
5. Antibiotic susceptibility results of MRSA
In this study, MRSA was 100% penicillin-resistant, except in 2006. The resistance rate of MRSA to erythromycin was 0%, 61.5%, 59.3%, and 50%; to gentamicin was 50%, 53.8%, 33.3%, and 35.7%, in 2006, 2009, 2015, and 2018; and to clindamycin was 0%, 38.5%, 33.3%, and 28.6%, in 2006, 2009, 2015, and 2018, respectively. The MRSA resistance rate to moxifloxacin, to which methicillin-susceptible S. aureus (MSSA) showed no significant resistance, was 0%, 23.1%, 29.6% and 21.4% in 2006, 2009, 2015, and 2018, respectively. Neither MSSA nor MRSA was resistant to vancomycin (Table 4).
|
Antibiotics |
No. of patients (%) |
||||
|
2006 |
2009 |
2012 |
2015 |
2018 |
|
|
Chloramphenicol |
0
(0) |
0
(0) |
- |
2
(2.2) |
- |
|
Ciprofloxacin |
- |
- |
- |
8
(9) |
4
(6.3) |
|
Clindamycin |
0
(0) |
0
(0) |
13
(30.2) |
17
(19.1) |
12
(18.8) |
|
Depomycin |
- |
- |
0
(0) |
0
(0) |
0
(0) |
|
Erythromycin |
0
(0) |
1
(6.3) |
17
(39.5) |
27
(30.3) |
17
(26.6) |
|
Gentamicin |
0
(0) |
25 |
9
(20.9) |
14
(15.7) |
12
(18.8) |
|
Linezolid |
0
(0) |
0
(0) |
0
(0) |
0
(0) |
1
(1.6) |
|
Moxifloxacin |
- |
0
(0) |
3
(7) |
8
(9) |
4
(6.3) |
|
Oxacillin |
0
(0) |
2
(12.5) |
13
(30.2) |
27
(30.3) |
14
(21.9) |
|
Penicillin
G |
1 (100) |
16 (100) |
41
(95.3) |
82
(92.1) |
53
(82.8) |
|
Rifampin |
0
(0) |
0
(0) |
0
(0) |
0
(0) |
2
(3.1) |
|
TMP-SMX |
0
(0) |
0
(0) |
0
(0) |
1
(1.1) |
0
(0) |
|
Vancomycin |
0
(0) |
0
(0) |
0
(0) |
0
(0) |
0
(0) |
|
Total |
1 (100) |
16 (100) |
43 (100) |
89 (100) |
64 (100) |
|
TMP-SMX,
trimethoprim-sulfamethoxazole |
|||||
|
Antibiotics |
No. of patients (%) |
||||
|
2006 |
2009 |
2012 |
2015 |
2018 |
|
|
Chloramphenicol |
0 |
0
(0) |
- |
1
(3.7) |
- |
|
Ciprofloxacin |
- |
- |
- |
8
(29.6) |
3
(21.4) |
|
Clindamycin |
0 |
0
(0) |
5
(38.5) |
9
(33.3) |
4
(28.6) |
|
Depomycin |
- |
- |
0
(0) |
0
(0) |
0
(0) |
|
Erythromycin |
0 |
0
(0) |
8
(61.5) |
16
(59.3) |
7
(50) |
|
Gentamicin |
0 |
1
(50) |
7
(53.8) |
9
(33.3) |
5
(35.7) |
|
Linezolid |
0 |
0
(0) |
0
(0) |
0
(0) |
0
(0) |
|
Moxifloxacin |
- |
0
(0) |
3
(23.1) |
8 (29.6) |
3
(21.4) |
|
Oxacillin |
0 |
2 (100) |
13 (100) |
27 (100) |
14 (100) |
|
Penicillin
G |
0 |
2 (100) |
13 (100) |
27 (100) |
14 (100) |
|
Rifampin |
0 |
0
(0) |
0
(0) |
0
(0) |
1
(7.1) |
|
TMP-SMX |
0 |
0
(0) |
0
(0) |
0
(0) |
0
(0) |
|
Vancomycin |
0 |
0
(0) |
0
(0) |
0
(0) |
0
(0) |
|
Total |
0 |
2
(100) |
13
(100) |
27
(100) |
14
(100) |
|
TMP-SMX,
trimethoprim-sulfamethoxazole |
|||||
For the treatment of bacterial infections, appropriate anti- biotics, which could be topical or systemic, are needed based on the nature of the skin lesion, kind of pathogens, and antimicrobial susceptibility. Because bacterial cultures and antimicrobial susceptibility tests in skin lesions take at least 2 to 3 days, the appropriate antibiotics are usually selected empirically according to clinical features as well as hospital admission history, history of recent antibiotic use, and recent information about antibiotic-resistant bacteria in the adjacent area3. Staphylococcus aureus is common in skin diseases, such as impetigo, soft tissue infections, abscess, furuncles, toxic shock syndrome, and staphylococcal scalded skin syn- drome. Most S. aureus species are penicillin-resistant. A pre- vious study3 of outpatients in 2001 reported that S. aureus was penicillin-resistant in 96.2% of the cases, 95.8% in a study2 in 2009, and 90.6% in this study. Thus, as in previous studies, S. aureus is highly penicillin-resistant. Since the first report of S. aureus resistance to penicillin in 1942, the fre- quency of resistant bacteria has remained steadily high, and penicillin seems to no longer be effective against S. aureus4, due to β-lactamase produced by S. aureus that hydrolyzes penicillin, preventing it from acting. To treat suspected S. aureus, methicillin, oxacillin, or cephalosporin antibiotics are used. However, since MRSA, which is resistant to these anti- biotics, was first reported in the 1960s, its prevalence has been increasing ever since5. According to the National Noso- comial Infections Surveillance report, the incidence of MRSA increased from 2.4% in 1975 to 29% in 19915. In Korea, the proportion of MRSA in inpatients was < 10% in the 1970s but increased sharply from 40% in 1980 to > 50% in the 1990s6-8. According to an outpatient study in 2009 in Korea, the proportion of MRSA was 7.5% in 2001 and 10.2% in 2006, which was lower than that of inpatients but showed an increasing prevalence. The outpatient study results showed that the proportion of MRSA increased from 12.5% in 2009, 30.2% in 2012, and 30.3% in 2015, respectively, and showed a slight decrease to 21.9% in 2018. Recent studies have shown that the incidence of both community-associated and hospital-associated MRSA have been reduced in some areas, which is consistent with the results of our study9,10. The reasons for the decreased incidence are not yet known but may be due to improved education for the prevention of hospital infections through the use of sterilization and con- tact precautions and improved early management of non- invasive infections9. In addition, changes in the virulence of circulating strains, including two clones of MRSA, ST22, and ST36, may have affected the prevalence of MRSA. Vanco- mycin and TMP-SMX can be used to treat cases of MRSA. The resistance rate of MRSA to TMP-SMX is currently < 10%, rendering MRSA to be susceptible to TMP-SMX. Vancomycin- resistant S. aureus has been reported in 14 cases in the United States to date11, and since vancomycin-intermediate S. aureus was first reported in Korea in 1999, 14 cases have been reported to date in Korea12. According to recent literature, no VRSA has been reported in Korea until now12. Therefore, based on the results of previous studies and the current study, MRSA is currently susceptible to these antibiotics.
Coagulase-negative staphylococci (CNS) are generally con- sidered to be normal flora rather than pathogens. In our investigation, CNS accounted for a considerable proportion (20.1%) of the identified bacteria, including those iden- tified as normal cutaneous microbiota, which may be due to insufficient sterilization before performing the culture or contamination during the examination, indicating the impor- tance of performing proper sterilization with povidone-iodine (betadine®) or chlorhexidine gluconate to obtain cultures uncontaminated by normal skin flora.
This study has some limitations. First, only outpatients were investigated, except for a few inpatients. The characteristics of the inpatients and the outpatients may be slightly different; hence, the microbiological characteristics of the inpatients may be different from the results of this study. Second, the data for the 2006 and 2009 studies are relatively small com- pared to those of the other years, so the result may be inaccurate as observed in the trend.
In conclusion, since MRSA was first reported in the 1960s, its prevalence has been increasing ever since. However, recent reports and the results of this study suggest that the incidence of MRSA has recently begun to decrease. The reason for such a decrease is not clear yet. Therefore, further epidemiological investigations are necessary to elucidate the cause of the decrease.
References
1. Yoon JS. Vancomycin resistance of Staphylococcus aureus in Korean primary hospitals. J Bacteriol Virol 2014;44: 305-310
2. Park JH, Byun JY, Lee DY, Lee JH, Yang JM, Lee ES. Trends of the bacterial skin infections of dermatology outpatients in 1996, 2001 and 2006. Korean J Dermatol 2009:47; 690-695
Google Scholar
3. Kim YJ. A study of prevalence and antibiotic susceptibilities of Staphylococcus aureus in the bacterial skin infection of dermatology outpatients. Korean J Dermatol 2001; 39:866-871
Google Scholar
4. Rammelkamp CH, Maxon T. Resistant of Staphylococcus aureus to the action of penicillin. Proc Soc Exp Bio Med 1942;51:386
Google Scholar
5. Panlilio AL, Culver DH, Gaynes RP, Banerjee S, Henderson TS, Tolson JS, et al. Methicillin-resistant Staphylococcus aureus in US hospitals, 1975-1991. Infect Control Hosp Epidermiol 1992;13:582-586
Google Scholar
6. Kim WJ. Nosocomial infection: change in the epidemiology. Korean J Med 1999;57:562-577
Google Scholar
7. Park SJ, Chong YS, Lee SY. Antibiotic susceptibility of clinical isolates of bacteria. Korean J Clin Pathol 1977;11: 119-125
8. Lee MK, Choi YS, Chong YS, Lee SY. Prevalence of methicillin-resistant Staphylococcus aureus and com- parison of susceptibility test method for its detection. Korean J Clin Pathol 1987;7:265-273
9. Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, et al. National burden of invasive methicillin- resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 2013;173:1970-1978
Google Scholar
10. Wyllie DH, Walker AS, Miller R, Moore C, Williamson SR, Schlackow I, et al. Decline of meticillin-resistant Staphylo- coccus aureus in Oxfordshire hospitals is strain-specific and preceded infection-control intensification. BMJ Open 2011;1:e000160
Google Scholar
11. McGuinness WA, Malachowa N, DeLeo FR. Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med 2017;90:269-281
Google Scholar
12. Park JW, Lee H, Kim JW, Kim B. Characterization of in- fections with vancomycin-intermediate Staphylococcus aureus (VISA) and Staphylococcus aureus with reduced vancomycin susceptibility in South Korea. Sci Rep 2019; 9:6236
Google Scholar
Congratulatory MessageClick here!