pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Kyoung Min Kang,Hong Kyun Kim,Hae Sook Lee,Kyung Eun Song,Sang Han Lee,Weon Ju Lee,Won-Kil Lee
http://dx.doi.org/10.17966/KJMM.2015.20.2.27 Epub 2015 September 20
Abstract
A 71-year-old man presented with pain in the left eye that revealed a 3×3 mm deep corneal stromal infiltrate, with a 2×2 mm epithelial defect. The patient started topical moxifloxacin, voriconazole 2%, and natamycin for 2 weeks. However, the treatment was not effective and the corneal infiltration worsened. Subsequently, the patient underwent therapeutic penetrating keratoplasty. Thick brown/gray mold colonies on Potato Corn Meal Tween 80 agar was isolated from excised corneal tissue and on slide culture many septated, and club-shaped ascospores were revealed.
Histological findings also showed numerous hyphae scattered in corneal tissue. A. alternata colonies were confirmed by 18S rRNA sequencing. Intracameral voriconazole was injected every other day for 2 weeks to eliminate remaining fungi on the deep corneal stroma. The remaining corneal infiltration was improved one month after the injection. During 5 months postoperative follow up, the infection did not recurred. In conclusion, deep corneal infection of A. alternata was effectively treated with intracameral voriconazole injection.
Keywords
Alternaria alternata Cornea culture Intracameral voriconazole injection
INTRODUCTION
Fungal keratitis is a potentially sight-threatening corneal infection due to the difficulties associated with its diagnosis and treatment[1]. Delayed diagnosis is common because the process is slow and initial symptoms are not severe. Gram and Giemsa staining of corneal scrapings is commonly used for initial and rapid identification of fungi. However, these methods have low sensitivity; recent studies have reported that positive identification of fungi occurs in approximately 27~33% of cases[2]. Fungal keratitis also poses a therapeutic challenge. There are limited commercially available antifungal agents; furthermore, they do not work as effectively as antibiotics for bacterial infections because of poor corneal penetration. The largest series of fungal keratitis has been reported from India, and the most common fungal isolates were Fusarium and Aspergillus species (36.6% and 30.4%, respectively), followed by dematiaceous fungi (15.7%)[3].
Alternaria species belong to a group of dematiaceous fungi and are ubiquitous in the environment as saprophytes of humans. Alternaria alternata is a common isolate that readily causes opportunistic infections in immunocompromised patients who have received long-term treatment with immunosuppressants such as steroid and tacrolimus. Alternaria species have been reported to cause cutaneous and subcutaneous infections, onychomycosis, sinusitis, visceral infections, and osteomyelitis[4]. However, corneal infection caused by Alternaria is less common and cases reporting infection in corneal tissue are rare. This present report describes a case of keratitis caused by A. alternata isolated from surgically excised corneal tissue.
CASE REPORT
A 71-year-old man presented with ocular pain and decreased visual acuity in the left eye. The patient had received pterygium surgery in the left eye 10 years previously. At presentation, best corrected visual acuity (BCVA) was 20/25 in the right eye and 20/70 in the left eye, and the intraocular pressure was 10 mmHg in both eyes. Slit-lamp biomicroscopy of the left eye revealed a nasal, juxtalimbal, 3×3 mm deep stromal infiltrate with a 2×2 mm epithelial defect. The feature of corneal infiltrate was irregular feathery margin and a dry rough texture. The sclera adjacent to the corneal lesion was white, avascular, and thin. Dark blue uveal tissue showed through the thin sclera and the conjunctiva was markedly injected (Fig. 1).
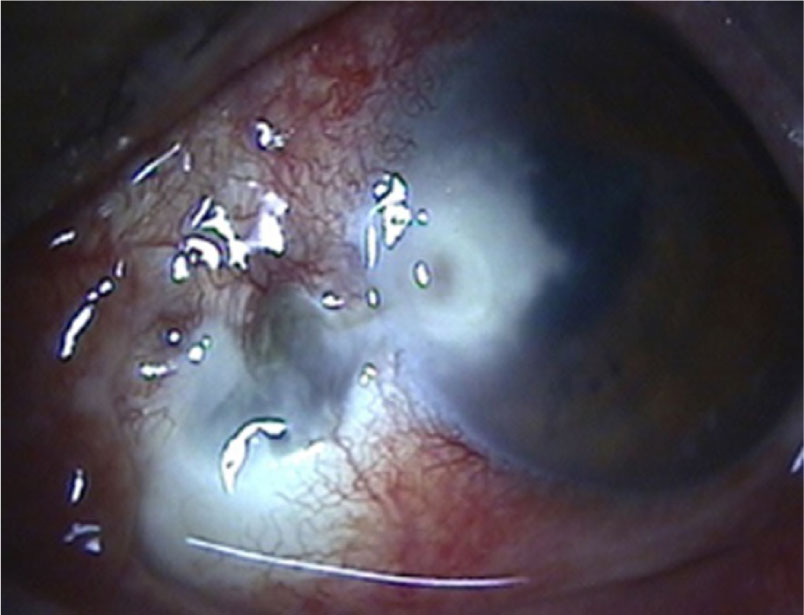
There was a severe anterior chamber reaction and the patient had a history of long-term topical corti-costeroid use to treat chronic ocular surface in-flammation of bare sclera originated from previous pterygium excision. Gram and Giemsa stainings of bacterial and fungal cultures from corneal scrapings were performed but did not reveal anything sug-gestive of fungal infection.
Clinical findings of insidious onset, irregular feathery margin and a dry rough texture, suggested fungal infection. The patient received initial empirical treatment for bacterial and fungal coverage; the treatment comprised hourly doses of topical moxifloxacin, voriconazole 2%, and natamycin and topical corticosteroid was discontinued. The cultures were obtained, no fungi could be identified after seven days. Within the first two weeks of intensive topical treatment, clinical signs and symptoms did not improve and the corneal infiltration progressed into deep stroma. Subsequently, the patient underwent therapeutic penetrating keratoplasty (PKP).

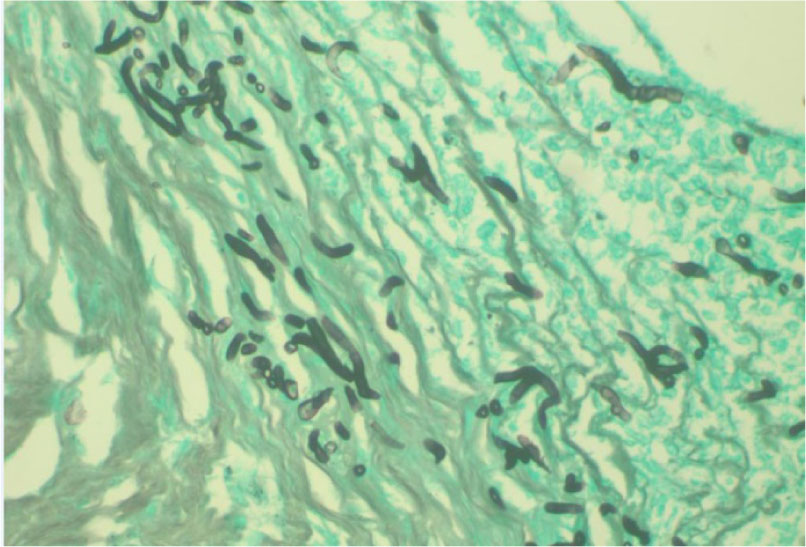
The infected cornea was removed and submitted to the clinical microbiology and surgical pathology laboratory for bacterial and fungal identification. Corneal grafts were sutured in place with 10~0 nylon. While the bacterial culture showed negative, four days after PKP was performed, fungal culture on Potato Corn Meal Tween 80 agar showed brown /gray mold colonies and a slide culture revealed many yellow-brown, septated, club-shaped ascospores, consistent with the findings for A. alternate (Figs. 2 & 3). H&E and Gomori methenamine silver stains also showed numerous hyphae scattered in the epithelial and stromal layers of corneal tissue (Fig. 4). The colonies of A. alternata in the culture were confirmed by 18S rRNA sequencing as following.

DNA was extracted with Genomic cell/tissue spin mini kit (Genotech, Daejeon, Korea). The 18S rRNA gene was amplified with the primer pair of TIS1 (TCCGTAGGTGAACCTGCGG). TIS4 (TCCTCCGCTTATTGATATGC) PCR was performed under the following conditions: initial denaturation at 95℃ for 5 min, followed by 30cycles of denaturation (95℃ for 45 sec) andannealing (55℃ for 45 sec), extension (72℃ for 45 sec), and a final extension step at 72℃ for 10 min. The PCR products were purified, and sequenced using an ABI 3730XL sequencer (Applied Biosystems, Foster City, CA, USA). All loci were sequenced in both the forward and reverse directions with the same primers as those used for the PCR. Sequence similarity searches were performed using the BLAST tool at the NCBI database (http://www.ncbi.nlm.nih.gov/blast) (Fig. 5).

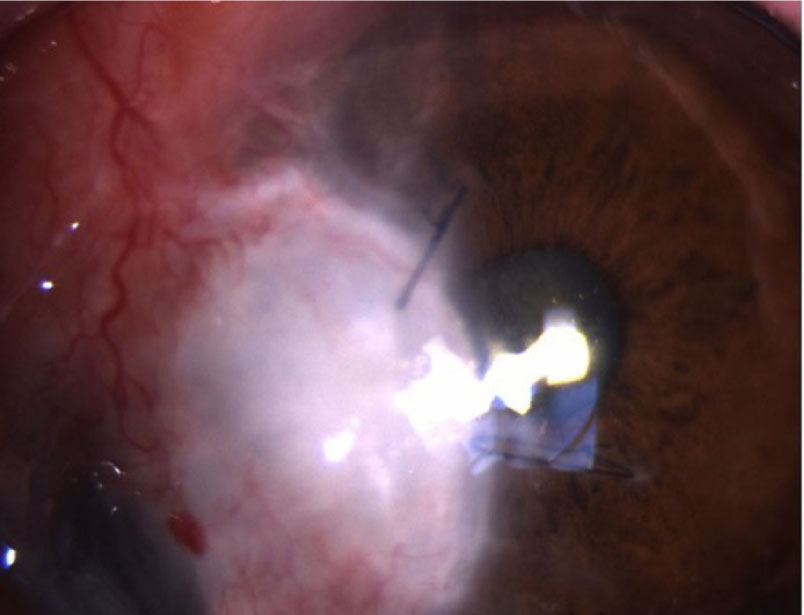
Although the transplanted corneal graft was well attached with clearance, deep corneal infiltration remained on the host's posterior corneal stroma. Intracameral voriconazole (100 μg/0.1 cc) and subconjunctival voriconazole (300 μg/0.3 cc) were injected under aseptic conditions. Intracameral and subconjunctival voriconazole were injected every other day for 2 weeks. One month after the injection of antifungal agents was administrated, the periphery of the corneal graft showed decreased infiltration. All topical antifungal agents were tapered and discontinued 2 months after surgery. The cornea remained clear five months postoperatively; there was no recurrence of fungal infection (Fig. 6) and BCVA in the left eye was 20/100.
DISCUSSION
Fungal keratitis is a sight-threatening disease because, in contrast to bacteria, fungi can penetrate Descemet's membrane and reach the anterior chamber. Once fungi reach the anterior chamber, elimination of fungi becomes difficult because topical antifungal agents have poor corneal penetration abilities. Furthermore, there are very few antifungal agents available commercially. Consequently, the treatment of fungal keratitis is difficult and the prognosis is poor.
Alternaria species area type of Dematiaceous fungus that produces melanin-like pigments. They are ubiquitous in the environment and frequently isolated from soil, plants, food, and air[5]. Opportunistic infections by these fungi can occur in immunosuppressant patients, particularly in bone marrow transplanted patients[6],[7]. Alternaria infection accounts for 3.3~8.7% of fungal keratitis cases[8],[9],[10],[11],[12]; it is less commonly associated with corneal infections. Hsiao et al. reviewed 25 previous case reports using the Medline database[13] and summarized the risk factors of Alternaria keratitis. Alternaria species infection was found to be associated with corneal trauma in 9 of 25 (36%) cases, previous corneal disease or surgery in 10 (40%) cases, and contact lens usage in 4 (16%) cases. Topical corticosteroids had been used in 14 of 25 (56%) of these cases, before diagnosis of fungal keratitis. Similarly, the patient in the present case had received long-term treatment with topical corticosteroids. The use of topical corticosteroid may therefore potentially result in the development and worsening of fungal keratitis. Mohd-Tahir et al. performed a retrospective review of all medical and microbiology records for all cases treated with fungal keratitis form January 2007 until December 2011[14]. They also reported the use of topical steroids was the second most common predisposing risk factor.
Fungal keratitis caused by A. alternata is a difficult form of microbial keratitis to diagnose and treat successfully. In these cases, early diagnosis and prompt treatment is necessary to achieve a successful outcome. Although Gram and Giemsa stainings can rapidly identify the fungi, these techniques have low sensitivities. Fungal cultures have higher sensitivities, but result in a longer time to diagnosis (>3 days). Alternative diagnostic methods with both high sensitivity and rapid diagnostic abilities are therefore required. In the present case report, both fungal culture and 18S rRNA sequencing from excised corneal tissue revealed A. alternata infection. Diagnosis of A. alternata infection is faster using polymerase chain reaction (PCR) compared to fungal culture. In this case, PCR using corneal scrapings taken from the first visit was not performed and the diagnosis was therefore delayed. The PCR method has the poten-tial to be a useful diagnostic tool because it enables early diagnosis and thereby facilitates prompt treat-ment.
In this case, the definitive diagnosis from corneal tissue was essential to choose the appropriate drug and its delivery method. Topical natamycin has been used for Alternaria keratitis, but it has limited effect because of its poor corneal penetration ability through intact epithelium. Ozbek et al. reported a case of Alternaria keratitis that was cured using systemic and topical voriconazole after treatment with topical natamycin and amphotericin B had failed5. However, in our presented case, deep seated infection could not treated with topical antifungal eyedrops. With the definitive diagnosis, we decided using intracameral voriconazole treatment. This present report also indicated that topical and intracameral use of voriconazole may be an effective treatment option for Alternaria keratitis resistant to natamycin and amphotericin B. Voriconazole has variable penetration abilities. The corneal epithelium serves as a barrier to the antifungal agent; therefore, in cases where the epithelium is intact, it is not possible to administer the agent to the desired level. The effectiveness of intracameral voriconazole injection for Alternaria keratitis has never been reported. In the present case, intracameral voriconazole injection with PKP resulted in the complete elimination of the fungi.
To our knowledge, this is the first case to report a successful outcome using intracameral vorico-nazole injection for Alternaria keratitis. In con-clusion, deep corneal infection of A. alternata was confirmed by laboratorial diagnosis and effectively treated with intracameral voriconazole injection.
References
1. Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol 2004;15:321-327
Google Scholar
2. Alfonso EC, Rosa RH, Miller D. Fungal keratitis. In: Krachmer JH, Mannis MJ, Holland EJ. editors. Cornea. 3rd ed. Vol. 1. New York: Mosby Elsvier Inc. 2011;1099-1122
3. Garg P, Gopinathan U, Choudhary K, Rao GN. Keratomycosis: clinical and microbiologic experience with dematiaceous fungi. Ophthalmology 2000; 107:574-580
Google Scholar
4. Arrese JE, Pie´rard-Franchimont C, Pie´rard GE. Onychomycosis and keratomycosis caused by Alternaria sp.: a bipolar opportunistic infection in a wood-pulp worker on chronic steroid therapy. Am J Dermatopathol 1996;18:611-613
Google Scholar
5. Ozbek Z, Kang S, Sivalingam J, Rapuano CJ, Cohen EJ, Hammersmith KM. Voriconazole in the management of Alternaria keratitis. Cornea 2006;25:242 -244
Google Scholar
6. Morrison VA, Haake RJ, Weisdorf DJ. The spectrum of non-Candida fungal infections following bone marrow transplantation. Medicine 1993;72:78-89
Google Scholar
7. Vartivarian SE, Anaissie EJ, Bodey GP. Emerging fungal pathogens inimmunocompromised patients: classification, diagnosis, and management. Clin Infect Dis 1993;7:487-491
Google Scholar
8. Chander J, Sharma A. Prevalence of fungal corneal ulcers in Northern India. Infection1994;22:207-209
Google Scholar
9. Tanure MA, Cohen EJ, Sudesh S, Rapuano CJ, Laibson PR. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea 2000;19:307-312
Google Scholar
10. Qiu WY, Yao YF, Zhu YF, Zhang YM, Zhou P, Jin YQ, et al. Fungal spectrum identified by a new slide culture and in vitro drug susceptibility using E test in fungal keratitis. Current Eye Research 2005;30:1113 -1120
Google Scholar
11. Jurkunas U, Behlau I, Colby K. Fungal keratitis: changing pathogens and risk factors. Cornea 2009; 28:638-643
Google Scholar
12. Wang L, Sun S, Jing Y, Han L, Zhang H, Yue J. Spectrum of fungal keratitis in central China. Clin Exp Ophthalmol 2009;37:763-771
Google Scholar
13. Hsiao CH, Yeh LK, Chen HC, Lin HC, Chen PY, Ma DH, et al. Clinical characteristics of Alternaria keratitis. J Ophthalmol 2014:536985. Epub 2014 Mar 20
Google Scholar
14. Mohd-Tahir F, Norhayati A, Siti-Raihan I, Ibrahim M. A 5-year retrospective review of fungal keratitis at hospital universiti sains malaysia. Interdiscip Perspect Infect Dis 2012;2012:851563
Google Scholar