pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Hye-Jin Ahn,Jin-Woo Lee,Min Kyung Shin
10.17966/JMI.2025.30.3.117 Epub 2025 October 02
Abstract
Keywords
Biofilm Nail plate Onychomycosis Scanning electron microscope
Onychomycosis treatment remains difficult, as antifungal therapies typically show low complete cure rates and high relapse rates. The pathophysiology of onychomycosis involves a complex process that includes fungal attachment, enzymatic breakdown of keratin, evasion of the host immune defenses, and gradual invasion of nail tissues, leading to significant structural and functional damage of the affected area. A nail plate affected by onychomycosis shows increased thickness but decreased tensile strength and density. Differences between healthy and infected toenails observed under a scanning electron microscope (SEM) have been documented previously. An SEM study of onychomycotic nail plates showed a ridged, tie-like ventral surface, with fungi on the underside of the toenail, evidence of penetrating the tissue corneocytes1. However, there is limited literature on the struc- tural characteristics of infected nail plates. In this study, SEM was used to observe the microscopic features of finger- and toenail plates from patients with onychomycosis, focusing on the structure of nail plates, fungal hyphae, spores, and biofilms within the infected tissues.
Following informed consent (KHUH Institutional Review Board approval 2024-04-033), fingernail and toenail plates were collected from three patients with a history of onycho- mycosis exceeding 3 years. After freeze-drying for 24 hours, SEM (TESCAN S8000 UHR FE-SEM, France) images were acquired.
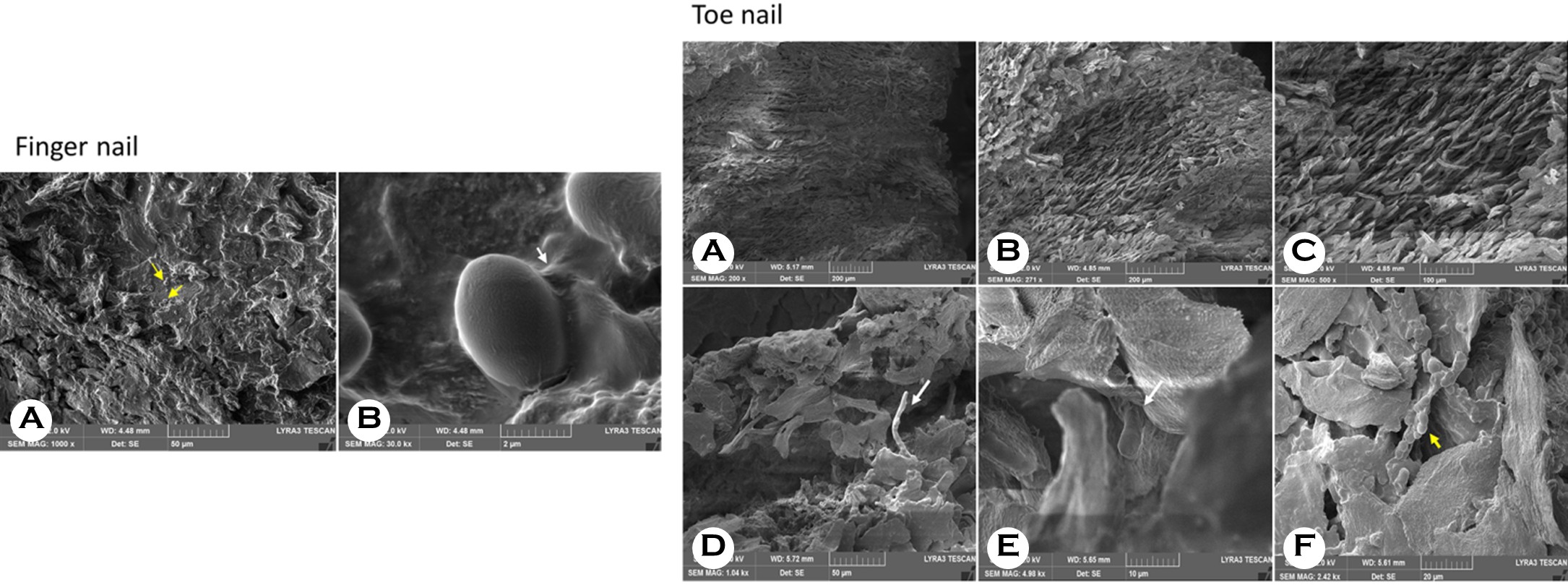
The ventral surface of the fingernail displayed irregularities and a rough, rugose texture. Numerous Candida spores, con- firmed by fungus culture, were observed (Fig. Finger nail A), including spores embedded within the biofilm matrix (Fig. Finger nail B). The toenail sample, over 2 mm thick, showed a ridged ventral nail plate with a tile-like appearance (Fig. Toe nail A), aligning with previously reported literature1. At higher magnification, the intermediate layer from the transverse section of the toenail plate specimen seemed to be empty, showing the presence of empty spaces (Fig. Toe nail B, C). As noted in earlier studies, this is seen as a structural change consistent with the fact that onychomycotic nails tend to be relatively thick yet porous barrier. The invading fungi can break down the intercellular matrix, leading to tissue that is less dense and has larger pores. Furthermore, damage to the intracellular matrix raises the tissue's permeability to topically applied chemicals, especially when using an aqueous vehicle. Another toenail plate sample was collected by carefully detaching a nail plate firmly affixed to the nail bed. In the ventral layer, hyphae penetrating the corneocytes (Fig. Toe nail D, E) were visible, along with spores attached to the uneven surface (Fig. Toe nail F). Fungal infections usually start in the keratinized tissue of the hyponychium and spread to the nail bed, ultimately affecting the nail plate. An acutely infected nail bed shows mild inflammation that can develop into a chronic infection, leading to complete dystrophy onychomycosis. In onychomycosis, the nail matrix can suffer secondary damage because the nail bed reacts to the fungal infection by becoming hyperkeratotic and thickened to eliminate the fungal pathogen. Infections affecting the nail matrix can result in permanent structural damage, causing nails to become abnormally thick and fragile, which are key characteristics of such damage, such as onychomycosis. Dermatophytes also penetrate the nail plate above, causing it to separate and weaken structurally. Most dermatophyte species first invade the ventral layer of the nail plate because it directly contacts the nail bed. The ridged, tile-like structure of the ventral surface creates a perfect environment for fungal growth. In our samples, we noted a significantly higher density of spores on the ventral layer, with some embedded within the biofilm matrix. These findings shed light on the difficulties in treating onychomycosis and its frequent recurrence. A prior study indicated that entirely eradicating dormant-phase spores of Trichophyton rubrum and T. mentagrophytes demands terbinafine concentrations 1,000 times higher than those effective during the active growth phase2. These findings suggest that dermatophytes in nail lesions can remain dormant even when systemic antifungal treatment is ongoing. Burkhart et al. proposed that biofilms play a role in the progression and poor outcomes of onychomycosis by supporting the continued adherence of dermatophytomas to the nail plate and making clinical management more challenging manage- ment3. Since biofilms are surrounded by an extracellular matrix that shields the microorganisms from host immune responses and antifungal treatments, it can be inferred that the biofilms in the samples partly contributed to the patient's treatment resistance. However, a limitation of this study is that only three patients were involved, and additional research with a larger sample size is necessary to confirm these results.
In conclusion, while the sample size was limited, this study revealed structural alterations in the infected nails along with numerous spores and biofilms, which could partly account for the ongoing difficulties in curing onychomycosis despite antifungal therapy.
References
1. Scherer WP, Scherer MD. Scanning electron microscope imaging of onychomycosis. J Am Podiatr Med Assoc 2004;94:356-362
Google Scholar
2. Seebacher C. Action mechanisms of modern antifungal agents and resulting problems in the management of onychomycosis. Mycoses 2003;46:506-510
Google Scholar
3. Burkharta CN, Burkhart CG, Gupta AK. Dermatophytoma: recalcitrance to treatment because of existence of fungal biofilm. J Am Acad Dermatol 2002;47:629-631
Google Scholar
Congratulatory MessageClick here!