pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Annapoorani SA,Moby Saira Luke,Priyadarshini Shanmugam
10.17966/JMI.2025.30.3.107 Epub 2025 October 01
Abstract
Strongyloidiasis is an asymptomatic condition caused by the nematode Strongyloides stercoralis, which is transmitted through the soil. It can go undetected for many years but can also be more severe in immunocompromised individuals. This paper reports on a case of Strongyloides stercoralis hyperinfection syndrome in a patient with chronic liver disease and type 2 diabetes mellitus. Upon screening of a stool sample sent to the microbiology lab, many rhabditiform larvae of Strongyloides stercoralis were discovered, a finding that was substantiated by the Department of Parasitology at JIPMER in Pondicherry, India. This was further confirmed through a conventional polymerase chain reaction test using Strongyloides stercoralis specific primers. During his stay, the patient was initially started on a piperacillin tazobactam injection. After the parasite was discovered, an oral course of tablet ivermectin 12 mg and tablet albendazole (400 mg daily) was added, after which the patient's condition improved drastically. He was discharged against medical advice due to personal reasons. On further follow-up, the patient was readmitted and the administration of tablet ivermectin and tablet albendazole was halted due to abnormalities in the liver function tests. After 2 days, he developed septic shock, refractory metabolic acidosis, protein-losing enteropathy and suspected drug-induced liver injury. Unfortunately, he later experienced a cardiac arrest and passed away. This case demonstrated that the early identification of Strongyloides infection is crucial to avert serious problems in at-risk patients, particularly those with immunocompromised conditions. Early identification and prompt antiparasitic treatments are enabled by detecting the parasites in faeces.
Keywords
Anemia Immunocompromised Infection Malnutrition Parasitology Protein-losing enteropathy Strongyloides stercoralis
An estimated 30-100 million people worldwide are in- fected with Strongyloidiasis, a disease brought on by the soil-transmitted helminth Strongyloides stercoralis1. Infections caused by Strongyloides stercoralis are less common than those caused by other intestinal nematodes. The parasite is widely distributed in hot, humid, and tropical areas, particularly in South-east Asia and South America. The parasite is primarily found among immigrants, refugees, travelers and military personnel in the Western World, especially those who have resided in the endemic areas. Strongyloides stercoralis may inhabit the intestine or exist in the soil as a free-living organism. The intestinal manifestations of Strongyloides infection ranges from asymptomatic to severe necrotizing bowel disease. Although the frequency of intestinal parasitic disease greatly decreased after World War II due to enhanced sanitation and infection control measures, Strongyloidiasis infection remains a concern.
Complicated Strongyloides infections occur in two forms: Hyperinfection syndrome and disseminated Strongyloidiasis. Hyperinfection syndrome is characterized by an exaggerated Strongyloides stercoralis life cycle without the dissemination of larvae beyond the typical migration routes like the lungs and gastrointestinal tract1. A disseminated infection indicates the presence of parasites outside their typical life cycle and in organs other than the skin, gastrointestinal system, or lungs2. The risk factors for developing disseminated Strongyloidiasis includes immunodeficiency, hematological malignancy, steroid administration, HTLV-1 disease, chronic alcoholism, renal failure and transplantation associated infections3. Individuals co-infected with human immunodeficiency virus are reported to experience unfavorable clinical outcomes4, including the weakening of immune protective responses caused by high alcohol consumption and elevated levels of endogenous cortisol, thereby promoting Strongyloides stercoralis auto-infection2,5, which is linked to a modest rise in morbidity and potential mortality.
A 36-year-old male was admitted to the emergency department for diffuse abdominal pain along with burning micturition, scrotal pain, and mild discomfort while breathing for 3 days, chronic vomiting for 3 months associated with abdominal distention, and significant weight loss of approxi- mately 15 kg within 6 months. The patient was a known case of chronic liver disease for over 3 months, type II diabetes mellitus on irregular medication for the past 6 months and suspected dermatomyositis (Fig. 1) for the past 2 years. Employed as a driver, he regularly obtained and ate food from external vendors. The patient had an uneventful family history. He was a chronic alcoholic and smoker for over 10 years, and his last binge had been 6 months prior to admission. The patient had developed allergic reactions to ceftriaxone, ondansetron and pantoprazole.
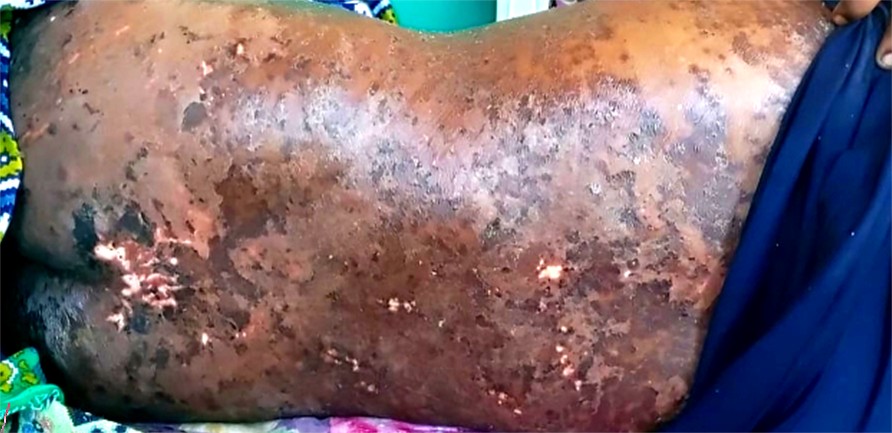
On physical examination, the patient was conscious, oriented, alert and afebrile with observed muscle wasting, mild anemia and bilateral pedal edema. There were no signs of lymphad- enopathy, cyanosis or icterus. His vital signs were normal on admission. Local examination revealed a well-defined, scaly, erythematous diffuse plaque over the trunk, face and neck. Systemic examination of the gastrointestinal tract revealed a distended abdomen with diffuse tenderness and no signs of any guarding or rigidity. The umbilicus was in midline and inverted. No palpable mass or organomegaly was observed and bowel sounds were sluggish. The cardiovascular system showed a normal sinus rhythm with a pulse rate of 110 beats/minute, blood pressure of 120/80 mmHg and S1S2 heard with no murmurs. The jugular venous pulse was not raised. The respiratory system showed equal bilateral air entry. An examination of his central nervous system showed no signs of any focal neurological deficits. His peripheral nervous system examination demonstrated normal tone, power, sensation and reflexes of all four limbs. An examination of the inguinal region revealed no palpable bulge while coughing or standing nor any post surgical scars were present. On genital examination, multiple hyperpigmented verrucous papules were noted over the prepuce, which could not be retracted. The scrotal region was inflamed and warty lesions were present on the fore skin with no visible sinuses.
The complete blood count indicated anemia with a hemo- globin level of 9.2 gm/dL and the peripheral smear revealed normocytic normochromic anemia along with a normal white blood cell count of 8,800 cells/mm3, exhibiting eosinophilia. The liver function tests showed decreased level of total protein, with a value of 3.5 g/dL, hypoalbuminemia with serum albumin of 0.9 g/dL, serum globulin of 2.5 g/dL, A/G ratio of 0.4:1, total bilirubin of 0.75 mg/dL, direct bilirubin of 0.437 mg/dL, aspartate transaminase of 27 U/L, alanine transaminase of 40 U/L, and alkaline phosphatase of 152 U/L. He had mild hyponatremia with a sodium level of 131 mEq/L, hypokalemia with a potassium level of 3.2 mEq/L and a reduced chloride level of 95 mEq/L. Further biochemistry investigations showed reduced folic acid levels of 5.57 ng/mL and increased vitamin B12 levels of 1,197 pg/ml. The iron profile showed a total of 29 μg/dl with a decreased total iron binding capacity of 28 μg/dl and ferritin had increased to 380.1 ng/ml.
A CT scan of the patient's chest and abdomen showed bilateral mild pleural effusion, primarily on the left side and consolidation in the posterior basal segment of both lower lobes. Mild ascites was noted with diffuse fatty infiltration of the liver with irregular borders was noted. Serological tests, including blood-borneviruses and ANA, were performed, and all were found to be negative. Two sets of blood samples were collected and sent to the microbiology department for culture and sensitivity testing, which yielded Staphylococcus other than Staphylococcus aureus (SOSa). Piperacillin tazobactam injection of 4.5 grams was given intravenously to the patient and his stool sample was sent for a stool routine and culture and sensitivity procedure.
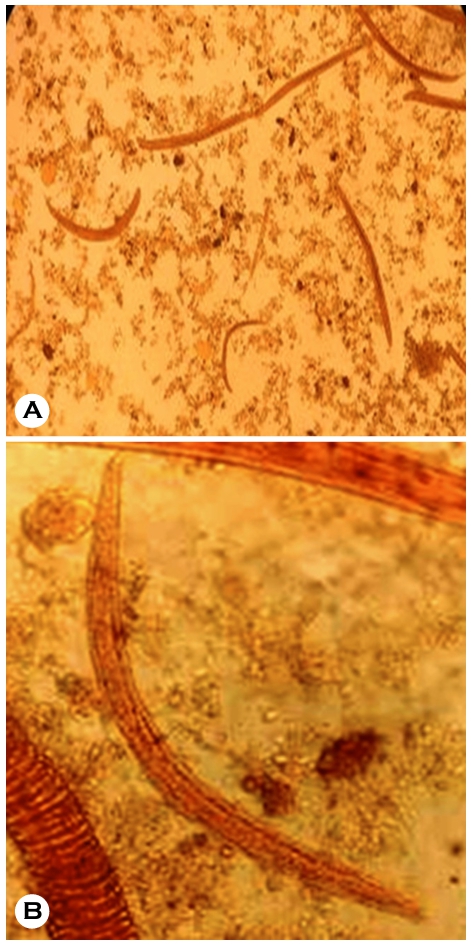
On macroscopic examination, the stool sample was brownish, semi-solid and non-mucoid in consistency. Micro- scopically, no RBCs or pus cells were observed. Many nema- tode larvae were noted, morphologically resembling the rhabditiform stage of Strongyloides stercoralis (Fig. 2). The consulting physicians started an oral course of tablet ivermectin (12 mg) and tablet albendazole (400 mg daily). A total of four doses were administered.
Due to personal reasons, the patient was discharged against medical advice after 10 days in the hospital. After 1 day, the patient was readmitted with worsening symptoms, intermittent vomiting and persistent scrotal swelling with mild abdominal distension. On re-admission, the patient was conscious and oriented with normal vital signs. Systemic examination of the gastrointestinal tract revealed a distended abdomen and mild diffuse tenderness present without any signs of guarding or rigidity. All other systems were within the normal limits. A skin biopsy was performed as per the Dermatologist's recommendations and the results were normal. Liver function tests showed elevated levels of Total Protein at 3.4 g/dL, Serum Albumin of 3 g/dL, Serum Globulin of 2.1 g/dL, A/G ratio of 0.6:1, a Total Bilirubin 2.47 mg/dL, Direct Bilirubin 1.532 mg/dL, AST of 56 U/L, ALT of 104 U/L, Alkaline Phosphatase of 346 U/L and Gamma-Glutamyl Trans- ferase was 259 IU/L. The Albendazole and Ivermectin therapy was therefore stopped, likely due to suspected Drug-induced liver injury. A General Surgery opinion was sought due to serous discharge from the patient's legs. He was prescribed Metronidazole (500 mg) intravenously (IV) twice daily and Topical antibiotics. On the morning of the fourth day after the patient's second admission, he developed a high fever and breathing difficulties with a SPO2 of 79% at room air and blood Glucose of 68 mg/dL. He was shifted to the Intensive Care Unit. Due to his low Glascow Coma Scale, the patient was intubated and connected to the ventilator and a central venous line and Ryle's tubes were placed. He was started on an injection course of Meropenem 1 gram and Vancomycin 1 gram IV stat and other supportive treatments. Following this, his blood pressure and sensorium improved. However, by that evening, the patient developed bradycardia and hypotension, eventually suffered a cardiac arrest and later succumbed. The final diagnosis was Anemia, Septic shock, Refractory Metabolic Acidosis, Protein-Losing Enteropathy, Strongyloides stercoralis hyperinfection, Suspected Drug-Induced Liver Injury With Type II Diabetes Mellitus and suspected Dermatomyositis.
The stool sample was sent to the Department of Parasi- tology at JIPMER in Pondicherry for species confirmation, and it was reported as the Rhabditiform stage of Strongyloides stercoralis. The larger genital primordium and shorter buccal capsule of rhabditiform larvae set them apart from hookworm larvae. Although its oesophagus is almost half as long as the body and the tail is notched, filariform larvae like wise resemble hookworm larvae. No concentration techniques were used in this study. Molecular detection for Strongyloides stercoralis was performed using a conventional Polymerase Chain Reaction (PCR) test with 2% Agarose Gel Electro- phoresis as per Nadarajan et al.6. The results are presented in Fig. 3 Primer sequences were selected as per Marquet et al. and Cunningham et al.7,8, who used specific primers for Strongyloides stercoralis McStrongy_F (Forward)5'-GATCA- TTCGGTTCATAGGTCGAT-3' and McStrongy_R (Reverse)5'-TACTATTAGCGCCATTTGCATTC-3'.
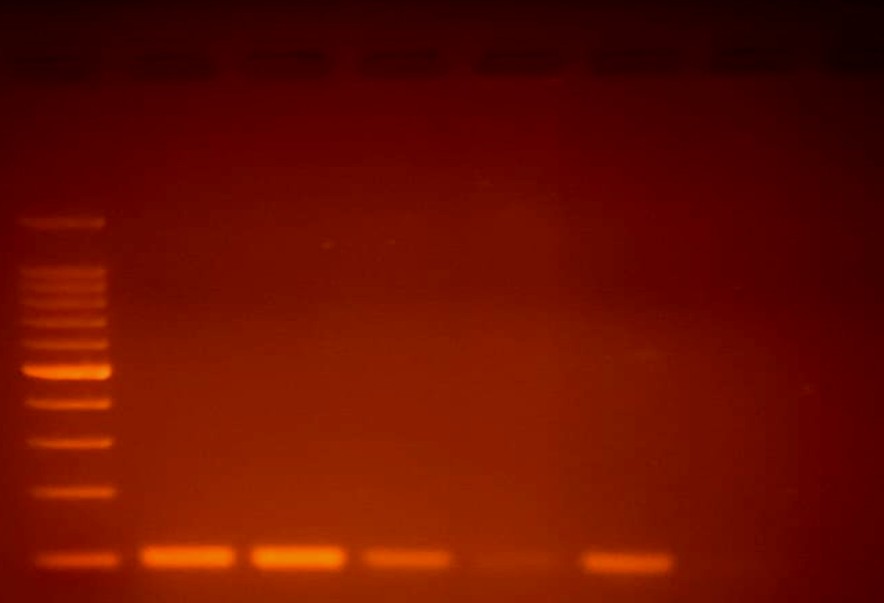
The DNA extraction procedure from the stool sample included cell lysis using lysis buffer and proteinase K to break down proteins and release DNA, which was incubated at 56℃ for 1 hour. Phase separation was performed using phenol-chloroform, followed by centrifugation at 12,000 rpm for 10 minutes. Cold isopropanol or ethanol was added, mixed and centrifuged to produce a pellet of DNA, which was washed with 70% ethanol and centrifuged again. The DNA was mixed with nuclease-free water and incubated at 55℃ for 10 minutes. The conventional PCR method was used. In short, an initial denaturation at 95℃ for 5 minutes was performed, followed by 30 cycles of amplification, denaturing, primer annealing, and primer extension at 94℃ × 30 seconds, 58℃ × 60 seconds, 72℃ × 30 seconds each. The final steps of PCR amplification involved raising the temperature to 72℃ × 7 minutes PCR products were observed using 2% agarose gel electrophoresis. Ethidium bromide (EtBr) staining was used for visualization under a UV trans-illuminator.
The life cycle of Strongyloides stercoralis is intricate and humans contract the infection when filariform larvae pre- sent in contaminated soil invades vulnerable host's skin or mucous membranes. The filariform stage is the infective form that enters the skin and reaches the heart and lungs via the circulatory system. In humans, identification is limited to adult females, and reproduction occurs asexually via parthenogenesis, a form of asexual reproduction in which spawning, growth and development occurs without fertil- ization8. Rhabditiform Strongyloides larvae have a short buccal cavity, a bulb oesophagus and a prominent genital primor- dium. The first-stage Rhabditiform larvae (L1) of Strongyloides stercoralis measure 180-380 μm in length and are distin- guished by a prominent genital primordium, a tripartite rhabditoid oesophagus that makes up about one-third of the body length and a short buccal canal. In second stage Rhabditiform larvae (L2), the bodily length is longer and characterized by a reduced oesophagus-to-intestine ratio. In contrast, the rhabditiform larvae of hookworm have a long buccal cavity and the esophagus-intestine junction is halfway down in filariform larvae of Strongyloides9.
Certain non infective rhabditiform larvae change into invasive filariform larvae before being excreted. Consequently, these larvae can reinfect the host by breaking through the intestinal wall or the skin around the anus10-12. The infectious third-stage filariform larvae (L3) of Strongyloides stercoralis are distinguished from hookworm larvae by an oesophagus-to-intestine ratio of 1:1, a notched tail and a length of up to 600 μm. Humans are infected by these L3 larvae through direct skin penetration, typically when bare feet contact contaminated soil. A single adult female can quickly reproduce through parthenogenesis by altering the host's immune response, resulting in increased autoinfection andspread13.
Clinically, a Strongyloides infection can present with gastro- intestinal complaints like diarrhea, constipation, abdominal pain, weight loss and epigastric discomfort, as well as cuta- neous signs like pruritic rash and larva currens. In chronic cases, the infection can be asymptomatic or only mildly symptomatic. In extreme situations, particularly in immunocompromised people, disseminated Strongyloidiasis and hyperinfection syndrome can occur, which are marked by widespread larval migration and can cause neurological problems, Gram-negative sepsis, Respiratory failure and even death with mor- tality rates close to 90%. In Brazil, various studies have shown that the prevalence of Strongyloides stercoralis infection among chronic alcoholics is approximately 20-15, nearly five times greater than in the general population16. A comparable outcome was discovered by Marques et al.17, showing a positive relationship between the severity of the alcoholism and the occurrence of the parasite in stools, regardless of the existence of liver cirrhosis17. The prevalence of intestinal parasites is higher among individuals who consume excessive alcohol due to inadequate hygiene. In a host with a competent immune system, the quantity of parasites and larvae produced is too low for detection, resulting in a significant likelihood of false negatives with standard stool-based techniques18,19.
Strongyloidiasis, often perceived as an unusual disease, primarily affects individuals in tropical areas or those with certain uncommon conditions that predispose them to in- fection. As the parasite can lead to autoinfection and hyper- infection, it is extremely important to raise awareness and increase the rates of diagnosis. Diagnosis of Strongyloides stercoralis can be achieved using a direct faecal smear or standard concentration procedures. It is essential to raise the clinical suspicion index for Strongyloides stercoralis infection in individuals with chronic alcoholism, those with diabetes, and those who are immunocompromised, as their weakened immune systems make them particularly susceptible to severe complications. Hyperinfection syndrome may result in severe, potentially fatal conditions, such as the widespread dissemi- nation of the parasite, respiratory complications and septic shock. Timely diagnosis and immediate intervention are essential for enhancing patient outcomes, particularly in high-risk populations.
Owing to its vague symptoms, this diagnosis is often overlooked and prognosis following late diagnosis is grim with a mortality rate of 15-87% even with treatment. Given the complexity of managing such cases, there is a pressing need for heightened awareness among health care providers and improved diagnostic and therapeutic strategies. With the increasing global prevalence of diabetes, alcoholism and immunocompromised conditions, further research into the interactions between these factors and Strongyloides stercoralis is essential for mitigating the risks associated with this parasitic infection.
References
1. Genta RM. Global prevalence of strongyloidiasis: Critical review with epidemiologic insights into the prevention of disseminated disease. Clin Infect Dis 1989;11:755-767
Google Scholar
2. Ganesh S, Cruz RJ Jr. Strongyloidiasis: A multifaceted disease. Gastroenterol Hepatol 2011;7:194-196
Google Scholar
3. Yoshida H, Endo H, Tanaka S, Ishikawa A, Kondo H, Nakamura T. Recurrent paralytic ileus associated with strongyloidiasis in a patient with systemic lupus erythema- tosus. Mod Rheumatol 2006;16:44-47
Google Scholar
4. Krolewiecki AJ, Lammie P, Jacobson J, Gabrielli AF, Levecke B, Socias E, et al. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis 2013;7:e2165
Google Scholar
5. Casado L, Rodriguez-Guardado A, Boga JA, Fernández-Suarez J, Martínez-Camblor P, Rodríguez-Perez M, et al. Use of serology in a systematic screening programme for strongyloidiasis in an immigrant population. Int J Infect Dis 2019;88:60-64
Google Scholar
6. Nadarajan A, Sivapushanam K, Ramasamy K, Jana D, Anbazhagan P. A simple and effective phenol-chloroform method of DNA extraction from mammalian faeces. Asian J Biotechnol Genet Eng 2022;5:1-8
Google Scholar
7. Marquet F, Mora N, Incani RN, Jesus J, Méndez N, Mujica R, et al. Comparison of different PCR amplification targets for molecular diagnosis of Strongyloides stercoralis. J Helminthol 2023;97:e88
8. Cunningham LJ, Stothard JR, Osei-Atweneboana M, Armoo S, Verweij JJ, Adams ER. Developing a real-time PCR assay based on multiplex high-resolution melt-curve analysis: a pilot study in detection and discrimination of soil-transmitted helminth and schistosome species. Parasitology 2018;145:1733-1738
Google Scholar
9. Castelletto ML, Akimori D, Patel R, Schroeder NE, Hallem EA. Introduction to Strongyloides stercoralis anatomy. J Nematol 2024;56:20240019
Google Scholar
10. Grove DI. Human strongyloidiasis. Adv Parasitol 1996;38: 251-309
Google Scholar
11. Grove DI, Blair AJ. Diagnosis of human strongyloidiasis by immunofluorescence, using Strongyloides ratti and S. stercoralis larvae. Am J Trop Med Hyg 1981;30:344-349
Google Scholar
12. Carroll SM, Karthigasu KT, Grove DI. Serodiagnosis of human strongyloidiasis by an enzyme-linked immuno- sorbent assay. Trans R Soc Trop Med Hyg 1981;75:706-709
Google Scholar
13. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology 2017;144:263-273
Google Scholar
14. Silva MLS, Inês Ede J, Souza AB, Dias VM, Guimarães CM, Menezes ER, et al. Association between Strongyloides stercoralis infection and cortisol secretion in alcoholic patients. Acta Trop 2016;154:133-138
Google Scholar
15. de Oliveira LC, Ribeiro CT, Demendes Dde M, Oliveira TC, Costa-Cruz JM. Frequency of Strongyloides stercoralis infection in alcoholics. Mem Inst Oswaldo Cruz 2002;97: 119-121
Google Scholar
16. Marques CC, Gomes MP, Gonçalves CS, Pereira FEL. Alcoholism and Strongyloides stercoralis: Daily ethanol ingestion has a positive correlation with the frequency of Strongyloides larvae in the stools. PLoS Negl Trop Dis 2010
Google Scholar
17. Bdioui A, Bchir A, Missaoui N, Hmissa S, Mokni M. Histo- pathological diagnosis of strongyloidiasis hyperinfection in Tunisian patient with hodgkin lymphoma: Case report. Ann Med Surg (Lond) 2021;66
Google Scholar
18. Marcos LA, Terashima A, Canales M, Gotuzzo E. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Rep 2011;13:35-46
Google Scholar
19. Teixeira MC, Pacheco FT, Souza JN, Silva ML, Inês EJ, Soares NM. Strongyloides stercoralis infection in alcoholic patients. Biomed Res Int 2016;2016:1-11
Congratulatory MessageClick here!