pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Abitha Evangelin Abraham,Priyadarshini Shanmugam,Suganeswari Ganesan
10.17966/JMI.2025.30.3.84 Epub 2025 October 01
Abstract
Background: Fungal keratitis is a severe, vision-threatening corneal infection predominantly observed in tropical and subtropical regions, where warm and humid conditions favor the proliferation of pathogenic fungi.
Objective: This study aimed to assess the epidemiological profile, causative agents, clinical presentation, and antifungal susceptibility patterns of patients with suspected fungal keratitis in Tamil Nadu, India.
Methods: A retrospective study was conducted at Chettinad Hospital and Research Institute from December 2019 to December 2023 involving 53 patients with suspected fungal keratitis. Clinical data, culture results, and antifungal susceptibility tests were analyzed.
Results: Of the 53 patients, 44 were culture-positive, mostly males (77.2%) from agricultural backgrounds. Trauma involving vegetative matter was the most common risk factor (55.5%), with the 40-50 years age group most affected. Common symptoms were redness (98%), pain (93%), and blurred vision (84%). Fusarium spp. were the predominant isolates (36.3%), followed by Aspergillus flavus (22.7%) and Curvularia spp. (15.9%). Antifungal susceptibility testing showed varying minimum inhibitory concentrations for amphotericin B, with Curvularia spp. exhibiting the greatest resistance (4 μg/mL).
Conclusion: The study highlights the diagnostic challenges of fungal keratitis due to its subtle onset and the limited efficacy of current antifungal therapies. Effective management requires early detection, accurate pathogen identification, and tailored antifungal treatment. High incidence in rural, diabetic, and occupationally exposed populations underscores the need for preventive public health strategies, including education, protective measures, and improved ophthalmic care access. The findings contribute valuable regional data supporting localized diagnostic and treatment protocols in endemic areas like Tamil Nadu.
Keywords
Epidemiological profile Fungal keratitis India Ophthalmic
Fungal keratitis is a critical ophthalmic condition that critically threatens vision, particularly in regions where warm and humid climates facilitate the growth of pathogenic fungi1. Characterized by corneal inflammation and ulceration, this condition is often associated with trauma to the eye, especially in individuals involved in agricultural activities, in whom contact with vegetative matter is common. The complexity of fungal keratitis lies in its diverse etiology, with a wide range of fungal species capable of causing infection, including Fusarium, Aspergillus, and Curvularia spp.2.
The diagnosis of fungal keratitis presents unique challenges because of the subtle clinical presentation of the condition in the early stages and the need for specialized microbiological investigations to confirm the presence of fungal pathogens. Delays in diagnosis or misdiagnosis can lead to rapid disease progression, resulting in significant corneal damage and potentially irreversible vision loss. Moreover, the treatment of fungal keratitis is often complicated by the limited availability of effective antifungal agents, the need for prolonged therapy, and the potential adverse effects of systemic and topical anti- fungal medications3. Given the high morbidity associated with fungal keratitis, understanding its epidemiological patterns and identifying key risk factors are essential for improving patient outcomes. In regions such as Tamil Nadu, where agri- culture plays a central role in the local economy, the incidence of fungal keratitis is particularly high, underscoring the need for targeted public health interventions4.
This study conducted a comprehensive evaluation of the epidemiological characteristics, causative organisms, and clinical outcomes of fungal keratitis among patients in a tertiary care center in Kelambakkam, Tamil Nadu, India. By systematically analyzing demographic data, risk factors such as diabetes and environmental exposure, and treatment responses, this research sought to elucidate the factors con- tributing to the disease's prevalence and improve the diag- nostic and therapeutic approaches to this potentially blinding condition.
Additionally, this study explored the distribution of specific fungal pathogens responsible for keratitis in this region in comparison with global trends. The findings will add to the existing body of knowledge on fungal keratitis and offer practical insights for clinicians in similar tropical and sub- tropical regions with a high disease burden.
This retrospective study was conducted at the Department of Microbiology, Chettinad Hospital and Research Institution, a tertiary care hospital in South India, from December 2019 to December 2023. The study commenced after obtaining approval from the Institutional Human Ethics Committee (reference ID: IHEC II/0102/21). After obtaining informed consent from the patients, 53 patients with suspected fungal keratitis who visited the OPD/IPD of Ophthalmology were included in the study. Samples were collected according to patient availability in the hospital. Patients with ocular in- fections other than fungal keratitis were excluded.
The following clinical variables were recorded: demographic data, medical history, risk factors such as history of ocular trauma with vegetative or non-vegetative material, contact lens use, prior ocular surgery, and clinical presentation. After a clinical assessment by the ophthalmologist, an experienced practitioner conducted a slit-lamp microscopic examination. Corneal scrapings were then collected under strict aseptic conditions using a sterile no. 15 Bard-Parker blade following the administration of topical anesthesia (4% lignocaine). The scrapings were taken from the edges and base of the ulcer, with the eyelids held open to prevent contamination from the lid margins or eyelashes. Scrapings were obtained from both central and peripheral regions of the ulcer using firm but gentle strokes.
Microscopic examination of ocular specimens involved subjecting a heat-fixed smear to Gram staining and pre- paring a wet mount with 10% KOH, which does not damage the cell walls of fungi, which remain intact and visible. This selective action is essential because it dissolves the cellular elements and increases the contrast between the fungi and the surrounding area, making it easier to identify fungal elements.
The collected material was inoculated onto culture medium, blood agar, and chocolate agar to culture bacterial patho- gens and Sabouraud's dextrose agar (SDA) to culture fungal pathogens. Cultures inoculated onto SDA were incubated at 25 and 37℃.
The inoculated media were examined daily for 8 weeks and discarded if no growth was observed. Fungal isolates were identified according to colony morphology on SDA, and lactophenol cotton blue (LPCB) mounts were prepared to identify filamentous fungi based on their microscopic characteristics, such as hyphal structure, septation, and spore morphology. Slide cultures were performed when necessary. To enhance identification accuracy, representative isolates were further confirmed using matrix-assisted laser desorption /ionization time-of-flight mass spectrometry (MALDI-TOF MS) at the Department of Microbiology, JIPMER (Puducherry, India). MALDI-TOF MS was performed using the Bruker Micro- flex LT/SH system (Bruker, Billerica, MA, USA). Fungal isolates were processed using the standard formic acid acetonitrile extraction protocol recommended by the manufacturer for filamentous fungi. Mass spectra were acquired and analyzed using Bruker Biotyper software (version 4.1, Bruker) with the Filamentous Fungi Library (version 3.0, Bruker). The identi- fication confidence score was interpreted as follows: ≥2.0, reliable species-level identification; 1.7-1.99, reliable genus-level identification; and <1.7, unreliable identification.
Only identifications with scores ≥1.7 were considered for reporting. Although common genera such as Fusarium, Aspergillus, and Curvularia were reliably identified because of their presence in the validated library, we acknowledge that MALDI-TOF MS has known limitations in distinguishing certain filamentous fungi species attributable to spectral overlap or database insufficiency. Molecular confirmation (e.g., ITS or β-tubulin gene sequencing) was not performed, which is a recognized limitation, especially for rare or cryptic species such as Basidiobolus and Cylindrocarpon spp.
1. Antifungal susceptibility testing
The antifungal susceptibility of the isolated pathogens was assessed according to Clinical and Laboratory Standards Institute (CLSI) guidelines outlined in document M27 for yeast and M38 for filamentous fungi. To prepare the fungal inocula, first, the fungal isolates were grown on SDA plates at 35℃ for 24-48 h. Then, suspensions of the fungal spores were created in sterile saline or distilled water, and the fungal concentration in each suspension was adjusted to achieve the 0.5 McFarland standard. To prepare antifungal solutions, stock solutions were created for the antifungal agents according to the manufacturer's instructions. Serial 2-fold dilutions of these agents were made in RPMI 1640 medium to achieve the required concentration ranges. In microdilution testing, 0.1 mL of growth medium were dropped into each well of a 96-well microtiter plate. Then, 100 μL of each antifungal dilu- tion were added to the wells, resulting in final concentrations of 0.03125-16 μg/mL, or other concentrations as appropriate for the specific antifungal agent, and then 100 μL of each prepared fungal inoculum were added to the wells. Control wells with a fungal inoculum but no antifungal agents and sterility control wells without a fungal inoculum were also included in the assay. The plates were covered and incubated at 35℃ for 24-48 h for yeast or up to 72 h for filamentous fungi. Fungal growth was visually assessed using a UV spec- trophotometer, and the optical density (OD) was measured at 530 nm. Epidemiological cutoff values (ECVs) were not applied to interpret the minimum inhibitory concentration (MIC) data, primarily because of the absence of established breakpoints for many of the isolated filamentous fungi. As such, the reported MICs are only descriptive and comparative, and they should not be used to infer clinical resistance or susceptibility.
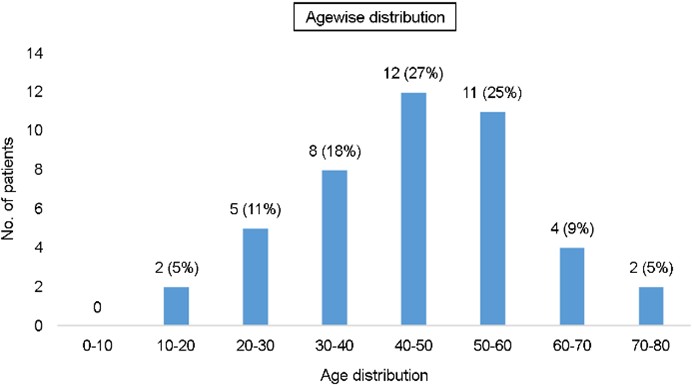
Of the 53 samples collected, 44 were culture-positive. Patients with culture-positive fungal keratitis patients were primarily male (34/44, 77.27%, Table 1). As presented in Fig. 1, patients with fungal keratitis were most commonly aged 40-50 years (28%), followed by 50-60 years (27%).
|
Demographic |
No. of patients |
|
Sex |
|
|
Male |
34 (77.3) |
|
Female |
10 (22.7) |
|
Source of infection |
|
|
Plant
source |
25 (56.8) |
|
Environment |
9 (20.5) |
|
Fabrication |
5 (11.4) |
|
Unkonw |
3 (6.8) |
|
Contact
lens |
2 (4.5) |
|
Occupation |
|
|
Farmer |
29 (65.9) |
|
Housewife |
9 (20.5) |
|
Student |
4 (9.1) |
|
Fabrication
worker |
2 (4.5) |
|
Association with DM/hypertension |
|
|
DM |
12 (27.3) |
|
DM
and hypertension |
2 (4.5) |
|
No |
30 (68.2) |
|
Presenting complains |
|
|
Redness |
43 (97.7) |
|
Pain |
41 (93.2) |
|
Blurring
of vision |
37 (84.1) |
|
Diminution
of vision |
35 (79.5) |
|
Watering |
22 (50.0) |
|
Discharge |
20 (45.5) |
|
White
opacity |
10 (22.7) |
|
Foreign
body sensation |
8 (18.2) |
|
Ophthalmic findings |
|
|
Epithelial
defect |
44 (100.0) |
|
Hypopyon |
41 (93.2) |
|
Satellite
lesions |
41 (93.2) |
|
Corneal
infiltrates |
40 (90.9) |
|
Corneal
edema |
38 (86.4) |
Concerning occupations, culture-positive patients were primarily farmers (65.9%). The primary source of infection was contact or trauma caused by vegetable matter (55.5%), with contact lenses being a less frequent cause (4.5%, Table 1). As listed in Table 1, symptoms experienced by patients with fungal keratitis included redness (98%), pain (93%), blurred vision (84%), decreased vision (79.5%), watering (50%), discharge (45.4%), white opacity (22.7%), and a foreign body sensation in the eye (18.1%). Most patients experienced reduced vision, redness, tearing, and pain at the infection site. In addition, 12 patients (27.27%) had co- incident diabetic mellitus, whereas two patients (4.5%) had coincident hypertension (Table 1).
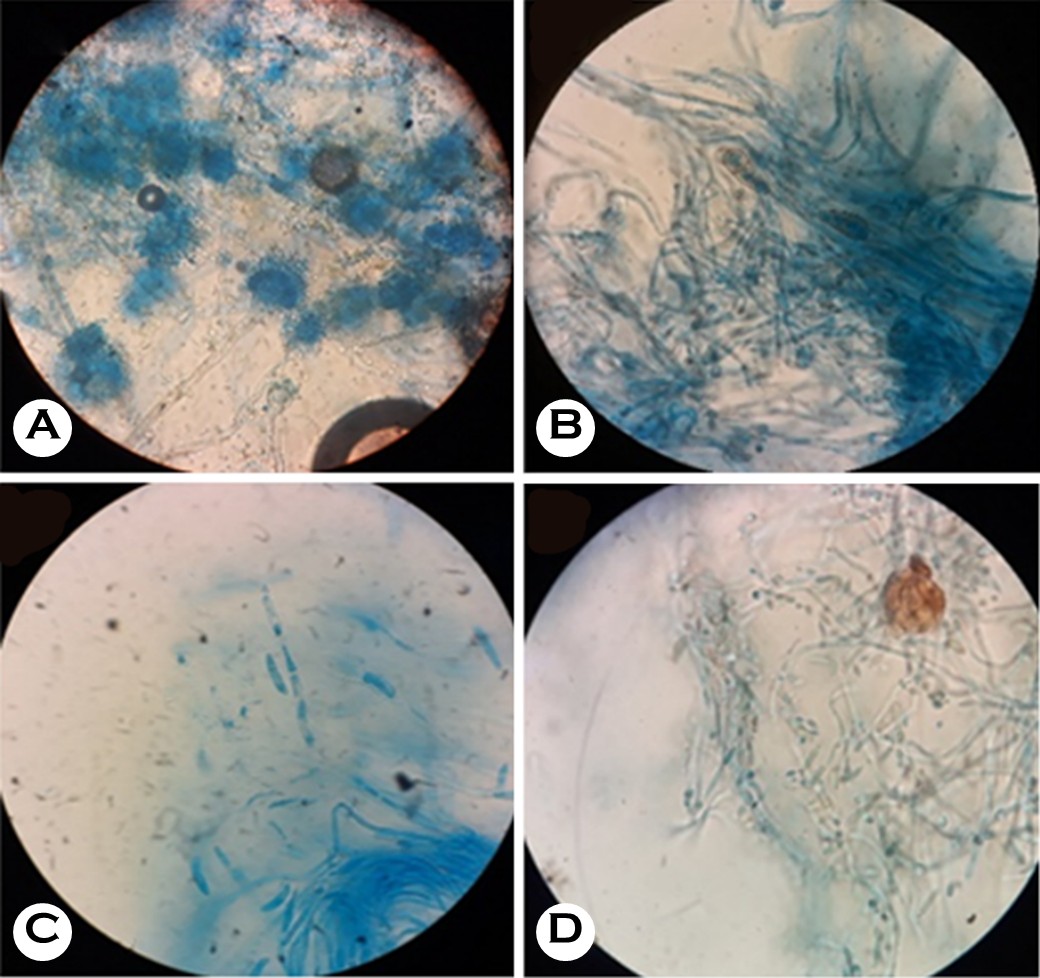
Fungal isolates were identified according to colony morph- ology on SDA, and LPCB mounts (Fig. 2) were prepared to identify filamentous fungi based on their microscopic characteristics. Based on MALDI-TOF MS, Fusarium spp. re-presented the most commonly isolated pathogens (16 isolates, 36.3%), followed by Aspergillus flavus (10 isolates, 22.7%) and Curvularia spp. (7 isolates, 15.9%). Meanwhile, Scedosporium spp. and Alternaria spp. were isolated from two patients each (4.5%), whereas Aspergillus fumigatus, Aspergillus niger, Cladosporium spp., Exserohilum turcicum, Penicillium spp., Basidiobolus spp., and Cylindrocarpon spp. were isolated from one patient each (2.2%, Table 2). The MIC is the lowest concentration of an antifungal agent that completely inhibits visible growth or leads to a significant decrease in OD relative to the control. The evaluation used amphotericin B and voriconazole as standard antifungal agents, with their results tabulated in Table 3. The results were presented as the mean ± standard deviation for quantitative variables and summarized as frequency (percentage) for categorical variables.
|
Fungal strains |
No.of isolates (%) |
|
Aspergillus fumigatus |
1 (2.27%) |
|
Alternaria |
2 (4.5%) |
|
Aspergillus flavus |
10 (22.72%) |
|
Aspergillus niger |
1 (2.27%) |
|
Cladosporium |
1 (2.27%) |
|
Curvularia spp. |
7 (15.9%) |
|
Exserohilum turcicum |
1 (2.27%) |
|
Fusarium spp. |
16 (36.36%) |
|
Penicillium |
1 (2.27%) |
|
Scedosporium spp. |
2 (4.5%) |
|
Basidiobolus spp. |
1 (2.27%) |
|
Cylindrocarpon |
1 (2.27%) |
|
Test isolates |
Amphotericin B |
|
Aspergillus fumigatus |
2 μg/ml |
|
Aspergillus flavus |
2 μg/ml |
|
Aspergillus niger |
0.5 μg/ml |
|
Curvalaria spp. |
4 μg/ml |
|
Fusarium solani |
2 μg/ml |
Fungal keratitis is a significant public health concern, particu- larly in tropical and subtropical regions where environmental factors and socioeconomic conditions contribute to its pre- valence5. The observed predominance of fungal keratitis among male patients in this study, particularly those engaged in agricultural activities, reflects broader epidemiological trends observed in similar geographical settings and gender-specific occupational exposures. Men, especially those working in agriculture, construction, and other outdoor occupations, are more frequently exposed to plant material, soil, and organic debris that harbor fungal spores6. This increased exposure heightens their risk of sustaining corneal injuries, which represent a common precursor to fungal infections. Moreover, cultural and social factors can influence healthcare-seeking behavior, with men potentially more likely to report or seek treatment for eye injuries. The age distribution, with a peak incidence in people aged 40-50 years, aligns with the results of several epidemiological studies in India, China, and Southeast Asia7. In these regions, middle-aged individuals represent the primary workforce in agriculture, leading to higher exposure to risk factors such as trauma from vegetation. The higher susceptibility in this age group could also be linked to cumu- lative exposure to environmental fungi over time, as well as age-related changes in ocular surface immunity and tear film stability, which can predispose individuals to infections8. Geographically, fungal keratitis is more prevalent in rural and agricultural regions in which environmental conditions, such as humidity and temperature, favor the growth and dispersion of fungi such as Fusarium and Aspergillus9,10. The study by Ghosh identified Fusarium spp. as the leading causative agents in this region, consistent with the environ- mental prevalence of these fungi in the soil and organic matter, particularly in tropical climates where fungal spores are more readily dispersed11. Seasonal variations also play a role, with Das et al. reporting that the incidence of fungal keratitis was higher during the rainy season, when agricultural activities peak and injuries related to plant debris are more common12. Socioeconomic factors are critical determinants in the epidemiology of fungal keratitis. In lower-income rural settings, limited access to healthcare, poor hygiene practices, and a lack of awareness about occupational safety and the serious nature of ocular injuries can delay diagnosis and treatment, leading to higher rates of fungal keratitis13. The reliance on traditional remedies and late presentation to healthcare facilities further complicate the management of fungal infections, often resulting in poorer outcomes in rural areas than in urban areas, which have better access to medical care14 (Chidambaram et al., 2018). The study by Bharathi et al. documented that corneal trauma is a well-established risk factor for fungal keratitis, with minor injuries often acting as the portal of entry for pathogenic fungi. The present study found that a large percentage (56%) of patients had a history of corneal injury, mainly caused by agricultural tools or plant materials, aligning with the findings of other research in comparable rural settings15. The role of trauma underscores the need for protective eyewear and education about eye safety in high-risk occupations. According to Prajna, in addition to trauma, pre-existing ocular surface diseases and the use of topical corticosteroids are risk factors for fungal keratitis16. Corticosteroids, commonly used to treat various ocular conditions, can suppress the local immune response, creating a favorable environment for fungal colonization and infection. The current findings should prompt a re-evaluation of the widespread use of topical corticosteroids in fungal keratitis-endemic regions. Fungal keratitis remains a global concern, with its incidence and etiology significantly varying by geographic location, climate, and population demographics. For instance, the incidence of fungal keratitis is lower in tem- perate regions, and the spectrum of causative fungi differs, with Candida spp. being more prevalent in temperate areas, in contrast to the dominance of filamentous fungi such as Fusarium and Aspergillus in tropical climates11. The global distribution and impact of fungal keratitis highlight the need for region-specific public health strategies, including aware- ness campaigns, protective measures for at-risk populations, and the development of accessible and effective antifungal treatments tailored to local epidemiology. Public health initia- tives should focus on preventive measures such as educating the rural workforce about the risks of corneal injuries and the importance of early medical intervention. Additionally, improving access to healthcare and promoting the use of protective eyewear in high-risk occupations could reduce the incidence of fungal keratitis and improve outcomes for affected individuals. Understanding the microbiological profile of fungal pathogens is crucial for effectively diagnosing and treating fungal keratitis. The microbiological profile encom- passes the types of fungi responsible for infections, their prevalence, and their antifungal susceptibility patterns. This profile provides insights into the specific disease-causing pathogens, informs treatment decisions, and facilitates the development of targeted therapeutic strategies17. In a prior study, Thomas identified Fusarium spp. as the most prevalent pathogens, accounting for 36.36% of the isolated fungi. Fusarium is widely found in soil and plant debris, and it frequently causes fungal keratitis, particularly in tropical and subtropical areas18. Fusarium spp. are highly pathogenic, and they are often associated with severe ocular infections because of their ability to invade corneal tissue aggressively. The filamentous nature of Fusarium allows it to penetrate deeper into the corneal stroma, leading to more severe disease outcomes19. Aspergillus flavus and Aspergillus niger were also significant pathogens in this study, representing 22.72% and 2.27% of the isolates, respectively. Aspergillus spp. are found throughout the environment, particularly in decaying plant material and soil. They are known to cause keratitis, often following trauma from organic matter18. Aspergillus flavus is particularly notorious for producing aflatoxins, which can exacerbate the virulence of infections20. According to Bharathi, Curvularia spp., accounting for 15.9% of the isolates in the present study, comprises another significant group of fungi associated with fungal keratitis. These fungi are found in soil, plant material, and decaying vegetation. Curvularia infections often occur in patients with a history of trauma from plant material, aligning with the findings in this study21. Curvularia spp. are known for their ability to cause chronic and recalcitrant infections, often requiring prolonged anti- fungal therapy15. Other isolated fungi included Alternaria spp., Cladosporium spp., Exserohilum turcicum, Penicillium spp., Scedosporium spp., Basidiobolus spp., and Cylindrocarpon spp., each representing a smaller percentage of the total isolates. Although these fungi are less common, their detec- tion underscores the diversity of fungal pathogens responsible for keratitis. For instance, Scedosporium spp. are often asso- ciated with more resistant infections, and they pose significant treatment challenges because of their inherent antifungal resistance22. Accurate identification of fungal pathogens in keratitis is achieved through a combination of clinical, micro- biological, and molecular techniques. Initial identification is often based on clinical presentation and slit-lamp micro-scopy, which can reveal characteristic features of fungal infections. However, definitive identification requires culture-based methods, in which fungi are isolated and identified according to their morphological and biochemical char- acteristics18,23. Molecular techniques, such as PCR and DNA sequencing, offer a more precise identification of fungi, and they can detect pathogens that are difficult to culture or identify using traditional methods24. These techniques are especially useful in identifying less common or emerging fungal pathogens, and they can provide insights into the genetic and epidemiological aspects of the infection21,25. Meanwhile, Ghosh noted that traditional antifungal treat- ments can have varying efficacy depending on the pathogen. For example, Fusarium and Aspergillus spp. are often resistant to certain antifungals, necessitating the use of alternative or combination therapies11. The development of resistance among fungal pathogens poses significant challenges in managing fungal keratitis and underscores the need for continuous surveillance and new antifungal agents. The epidemiological patterns, risk factors (e.g., trauma caused by vegetative material), and predominant fungal species reported in this study are consistent with previous findings from India and other tropical regions. Although the use of MALDI-TOF MS and the identification of rare fungal species (e.g., Basidiobolus spp., Scedosporium spp., Cylindrocarpon spp.) offer some novel insight, the overall findings might not represent a substantial deviation from established regional data. However, this study provides updated evidence from a 4-year post-pandemic period, which could reflect evolving trends in fungal keratitis etiology and antifungal susceptibility. The diverse microbiological profile of fungal pathogens high- lights the necessity for individualized treatment strategies based on the specific fungi isolated from patients. Empirical treatment with broad-spectrum antifungals is often employed initially, but definitive therapy should be tailored according to the susceptibility profiles of the isolated pathogens26.
Some limitations of this study should be acknowledged. This study was conducted retrospectively at a single tertiary care center, and it included a relatively small number of patients (n = 53), which might limit how broadly the findings can be applied to other settings or populations. Although we used MALDI-TOF MS, a rapid and increasingly popular method, for fungal identification, the reliability of this tech- nique depends heavily on the reference database. Because molecular confirmation (such as ITS or β-tubulin sequencing) was not performed, there might be some uncertainty in the species-level identification of certain rare or uncommon fungi. In terms of antifungal susceptibility testing, although we followed CLSI guidelines (M27 for yeast and M38 for fila- mentous fungi), established clinical breakpoints or ECVs could not be applied for most isolates, as these data are not currently available for many filamentous fungi. Thus, the MIC data presented should be viewed as descriptive rather than predictive of clinical outcomes. Finally, although our findings on the most common fungal pathogens and associated risk factors are consistent with previous results from tropical regions, this study contributes updated local data, including rarely reported Cylindrocarpon spp., Scedosporium spp., and Syncephalastrum spp.
References
1. Oliveira Dos Santos C, Kolwijck E, van Rooij J, Stoutenbeek R, Visser N, Cheng YY, et al. Epidemiology and clinical management of Fusarium keratitis in the Netherlands, 2005-2016. Front Cell Infect Microbiol 2020; 10:133
Google Scholar
2. Iyer SA, Tuli SS, Wagoner RC. Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens 2006; 32:267-271
Google Scholar
3. Kredics L, Narendran V, Shobana CS, Vágvölgyi C, Manikandan P. Filamentous fungal infections of the cornea: a global overview of epidemiology and drug sensitivity. Mycoses 2015;58:243-260
Google Scholar
4. Chidambaram JD, Venkatesh Prajna N, Srikanthi P, Lanjewarin S, Shah M, Elakkiya S, et al. Epidemiology, risk factors, and clinical outcomes in severe microbial keratitis South India. Ophthalmic Epidemiol 2018;25:297-305
Google Scholar
5. Raj N, Vanathi M, Ahmed NH, Gupta N, Lomi N, Tandon R. Recent perspectives in the management of fungal keratitis. J Fungi (Basel) 2021;7:907
Google Scholar
6. Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and labora- tory diagnosis of fungal keratitis: a three-year study. Indian J Ophthalmol 2003;51:315-321
Google Scholar
7. Tawde Y, Singh S, Das S, Rudramurthy SM, Kaur H, Gupta A, et al. Clinical and mycological profile of fungal keratitis from North and North-East India. Indian J Ophthalmol 2022;70:1990-1996
Google Scholar
8. Singh M, Gour A, Gandhi A, Mathur U, Farooqui JH. Demographic details, risk factors, microbiological profile, and clinical outcomes of pediatric infectious keratitis cases in North India. Indian J Ophthalmol 2020;68:434-440
Google Scholar
9. Kim LN, Karthik H, Proudmore KE, Kidd SE, Baird RW. Fungal keratitis: epidemiology and outcomes in a tropical Australian setting. Trop Med Infect Dis 2024;9:127
Google Scholar
10. Castano G, Elnahry AG, Mada PK. Fungal keratitis. StatPearls - NCBI Bookshelf 2024 Feb 12. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493192/
11. Ghosh AK, Gupta A, Rudramurthy SM, Paul S, Hallur VK, Chakrabarti A. Fungal keratitis in North India: spectrum of agents, risk factors and treatment. Mycopathologia 2016;181:843-850
Google Scholar
12. Danielescu C, Stanca HT, Iorga RE, Darabus DM, Potop V. The diagnosis and treatment of fungal endophthalmitis: an update. Diagnostics (Basel) 2022;12:679
Google Scholar
13. Burton MJ, Pithuwa J, Okello E, Afwamba I, Onyango JJ, Oates F, et al. Microbial keratitis in East Africa: why are the outcomes so poor? Ophthalmic Epidemiol 2011;18: 158-163
Google Scholar
14. Prajna NV, Krishnan T, Rajaraman R, Patel S, Srinivasan M, Das M, et al. Effect of oral voriconazole on fungal keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II): a randomized clinical trial. JAMA Ophthalmol 2016;134: 1365-1372
Google Scholar
15. Soleimani M, Izadi A, Khodavaisy S, Dos Santos CO, Tehupeiory-Kooreman MC, Ghazvini RD, et al. Fungal keratitis in Iran: risk factors, clinical features, and myco- logical profile. Front Cell Infect Microbiol 2023;13: 1094182
Google Scholar
16. Hoffman JJ, Burton MJ, Leck A. Mycotic keratitis - a global threat from the filamentous fungi. J Fungi (Basel) 2021; 7:273
Google Scholar
17. Asanka Sanjeewa KK, Jayawardena TU, Kim HS, Kim SY, Shanura Fernando IP, Wang L, et al. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflam- mation in macrophages blocking TLR/NF-κB signal path- way. Carbohydr Polym 2019;224:115195
Google Scholar
18. Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect 2013; 19:210-220
Google Scholar
19. Belskaia KI, Obrubov AS. Pathogenesis and clinical features of fungal keratitis (review). Ophthalmology in Russia 2021;18:12-19
Google Scholar
20. Bălășoiu AT, Bălășoiu M, Zlatian OM, Ghenea AE. Bacterial and fungal keratitis in a tertiary care hospital from Romania. Microorganisms 2024;12:787
21. Manikandan P, Abdel-Hadi A, Singh YRB, Revathi R, Anita R, Banawas S, et al. Fungal keratitis: epidemiology, rapid detection, and antifungal susceptibilities of Fusarium and Aspergillus isolates from corneal scrapings. Biomed Res Int 2019;2019:6395840
Google Scholar
22. Awad R, Ghaith AA, Awad K, Mamdouh Saad M, Elmassry AA. Fungal keratitis: diagnosis, management, and recent advances. Clin Ophthalmol 2024;18:85-106
Google Scholar
23. Saleh A, Pirouzifard M, Khaledabad MA, Almasi H. Kappa-carrageenan based active nanocomposite films incorp- orated with nanohalloysite and niosome-encapsulated Lippia citriodora essential oil: preparation and character- ization. Appl Food Res 2025;100885
Google Scholar
24. Niu L, Liu X, Ma Z, Yin Y, Sun L, Yang L, et al. Fungal keratitis: pathogenesis, diagnosis and prevention. Microb Pathog 2019;138:103802
Google Scholar
25. Ara T, Shafi S, Ghazwani M, Mir JI, Shah AH, Qadri RA, et al. In vitro potent anticancer, antifungal, and anti- oxidant efficacy of walnut (Juglans regia L.) genotypes. Agronomy 2023;13:1232
Google Scholar
26. Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev 2003;16:730-797
Google Scholar
Congratulatory MessageClick here!