pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Priyadharshini Sonai,Priyadarshini Shanmugam,John Maria Louis P,Alice Peace Selvabai R
10.17966/JMI.2025.30.3.75 Epub 2025 October 01
Abstract
Background: Streptococcus pneumoniae (S. pneumoniae) is a major cause of illness and mortality globally across age groups. WHO has categorized Streptococcus pneumoniae as an agent of top concern due to its high disease burden and growing resistance to commonly used antibiotics. This study holds significant clinical and public health implications and seeks to strengthen antibiotic stewardship efforts by providing data-driven insights into resistance trends to ensure more effective management of pneumococcal infections.
Objective: To determine the molecular characteristics and resistance profile of Streptococcus pneumoniae isolated from respiratory samples of LRTI patients.
Methods: This observational prospective study was conducted over one year in the Clinical Microbiology Lab at Chettinad Hospital and Research Institute, Tamil Nadu, to analyze the microbiological and molecular characteristics of isolates (Streptococcus pneumonia) from 50 patients having lower respiratory airway infections (LRTIs), including pneumonia.
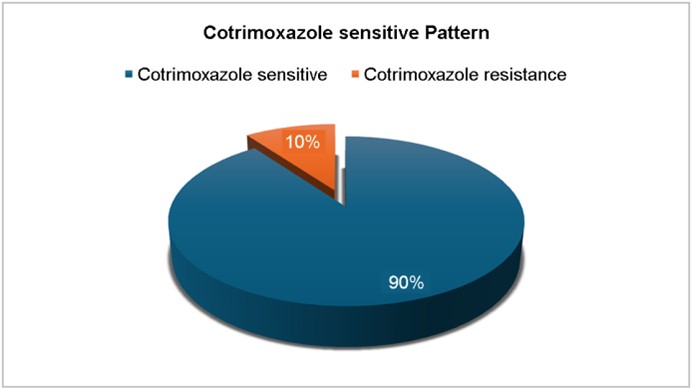
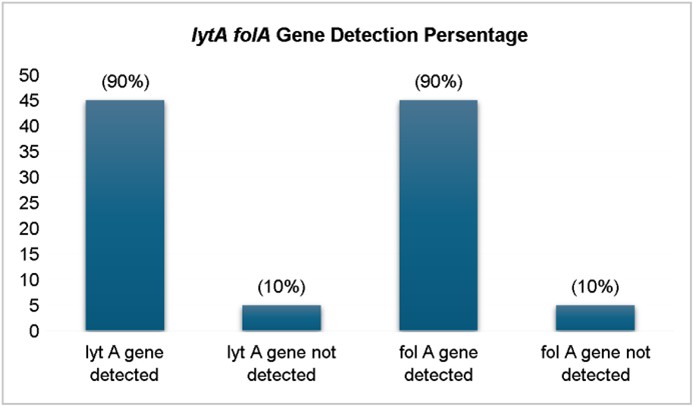
Results: Analysis of the antimicrobial resistance patterns of 50 Streptococcus pneumoniae isolates from patients with LRTIs revealed high resistance to cotrimoxazole (90%), tetracycline (66%), and erythromycin (60%). Every single isolate showed sensitivity to a range of antibiotics, including cefotaxime, vancomycin, azithromycin, linezolid, and cefazolin. Polymerase chain reaction (PCR) interpretation for the lytA and folA genes provided additional insights into pneumococcal pathogenesis and antimicrobial resistance. The lytA gene, which encodes autolysin and is essential for cell wall degradation, was detected in 45/50 (90%) isolates, confirming its virulence. The folA gene, associated with resistance to cotrimoxazole, was also detected in 45/50 (90%) isolates, correlating well with the observed resistance patterns.
Conclusion: Among the 50 isolates, 76% originated from sputum, and were obtained predominantly from female patients (60%) and those aged 19-60 years (46%). High resistance rates were noted for cotrimoxazole (90%), tetracycline (66%), and erythromycin (60%). All isolates remained fully susceptible to cefotaxime, vancomycin, linezolid, azithromycin, and cefazolin. The folA gene was detected in all cotrimoxazole-resistant strains, confirming
Keywords
Antibiotic-resistant pneumococci Cotrimoxazole resistance Genotypic study Lower respiratory infection Molecular testing Pneumococci Virulence genes
Streptococcus pneumoniae (S. pneumoniae) or pneumo- cocci are major causes of illness and death globally across age groups. To date, over 90 distinct serotypes have been identified, distinguished by variations in their capsular polysaccharides, the primary virulence component of the bacterium1. Serotype distribution varies by geographic region, age group, and clinical manifestation, making it essential for vaccine develop- ment and public health interventions to track the circulating serotypes2. The likelihood of vaccine-serotype illnesses has been significantly decreased by pneumococcal conjugate vaccinations (PCVs), where non-vaccine serotypes (NVTs) have become increasingly prevalent, contributing to continued pneumococcal disease burden3.
The development and spread of drug-resistant pneumo- cocci are facilitated by the injudicious use of antibiotics, limiting treatment options and increasing disease severity. These strains exhibit decreased response to β-lactam anti -microbial agents. Other antibiotic classes, including macrolides, tetracyclines, and fluoroquinolones, have also seen increasing resistance rates, further complicating treatment strategies4. Additionally, horizontal gene transfer through transformation, transduction, and conjugation has played an essential role in the spread of resistance determinants among pneumococcal populations5.
India bears a substantial burden of pneumococcal dis- eases, particularly among children less than 5 years of age. As per the Global Burden of Disease study, an important cause of mortality in this age group in India is pneumococcal pneumonia6. The inclusion of PCV13 in India's Universal Immunization Programme has been a crucial step in reducing pneumococcal disease burden; however, regional variations in vaccine coverage, serotype prevalence, and antimicrobial resistance patterns remain significant challenges7.
Surveillance studies in India have reported an alarming increase in penicillin-resistant, multidrug-resistant isolates of Streptococcus pneumoniae8. However, comprehensive data on the genotypic diversity of pneumococcal strains circulating in India and their characterization by molecular methods and resistance profiles for antibiotics remain limited. The lack of nationwide surveillance programs has hindered efforts to develop effective treatment guidelines and vaccination strategies tailored to the Indian population. This study examined the microbiological and molecular aspects and the drug resistance and virulence factors of, laboratory-verified Streptococcus pneumoniae strains from patients suffering from lower respiratory tract infections (LRTIs).
1. Study design
The study was designed as a prospective observational study. After obtaining institutional ethical committee clearance the study was conducted in the Department of Microbiology at Chettinad Hospital and Research Institute for a period of one year.
2. Sample size
50 clinical isolates of Streptococcus pneumoniae strains in respiratory samples from individuals with LTRIs. The sample size was determined based on prevalence rates, previous studies, and feasibility within the study duration. For a com- parison of resistance rates among different subgroups (e.g., male vs. female, sputum vs. other samples), a sample size of 50 provides adequate power for detecting moderate-to-large differences (Cohen's d ≈ 0.6-0.8). A power analysis was performed using G Power to assess the adequacy of the sample size (n = 50) for statistical comparisons. For a chi-square test to evaluate the associations between gene prevalence and antimicrobial resistance (effect size w = 0.5, α = 0.05, power = 0.80), the required sample was 45-50, supporting the sufficiency of our dataset. Similarly, for group comparisons using t-tests, a sample of 50 allows the detection of moderate-to-large differences in resistance patterns and clinical characteristics. Therefore, the chosen sample size is statistically justified for the meaningful microbiological and molecular analysis of S. pneumoniae isolates in LRTI patients.
3. Inclusion criteria
Patients of all age groups, both male and female, with LRTIs, from whom Streptococcus pneumoniae had been isolated.
4. Exclusion criteria
Samples from COVID-positive patients and Hemophilus influenzae and infectious agents other than Streptococcus pneumonia were excluded in this study.
5. Study procedure
This study analyzed 50 non-repetitive Streptococcus pneumoniae samples isolated from samples taken from respiratory tract patients diagnosed with LRTI. The isolates were subjected to phenotypic tests, followed by molecular characterization to detect genes specific for virulence and antibiotic resistance by conventional multiplex polymerase chain reaction (PCR). Samples were collected from patients who presented with LRTI after informed consent was obtained. The types of specimens included: sputum, endotracheal aspirate (ETA), bronchoalveolar lavage (BAL) fluid, and throat swabs (patients with throat pain and LRTI symptoms).
1) Sample processing
Sputum, ETA, BAL, and throat swab collections were per- formed following strict protocols to ensure sample quality. Patients were instructed to rinse their mouth before sputum collection and then cough deeply into a sterile container. For ETA, a sterile suction catheter was used, while BAL involved bronchoscope-guided lavage with sterile saline. Throat swabs were collected with sterile swabs while avoiding contamination from the oral cavity. Samples were promptly transported to the laboratory, with those delayed refrigerated at 2-8℃.
2) Gram stain from the clinical sample
A thin, uniform smear was prepared by slide, free of grease and clean, using an appropriate clinical specimen under aseptic conditions. The smear was dried in air and fixed by heat by gently passing the slide through a flame to ensure fixation of the microbial cells without distorting their morphology. Gram stain was performed as per the Hucker's modification method: 1 minute crystal violet (primary stain) was applied to the smear, followed by 1 minute with Gram's iodine, another washing, 2 seconds with decolorizer acetone, immediate washing, then 1 minute with counterstain safranin. This was followed by a final wash, drying, and viewing under the oil immersion objective. Findings showed Gram-positive, lanceolate-shaped diplococci with a clear halo indicating a capsule.
Examination of the culture, 5% sheep blood agar using 5 to 10% CO2, showed the growth of alpha hemolytic colonies through a blanching effect on chocolate agar. Sensitivity for optochin and solubility tests for bile were also performed to confirm the isolates as pneumococci.
Disk diffusion by Kirby Bauer for susceptibility testing for antibiotics was performed using discs impregnated with the following antibiotics: cefotaxime 30 mcg, vancomycin 30 mcg, azithromycin 15 mcg, linezolid 30 mcg, cefazolin 30 mcg, clindamycin 2 mcg, cotrimoxazole 23.75/1.25 mcg, tetra- cycline 30 mcg, and erythromycin 15 mcg. A lawn culture of the pneumococcal isolates was made on Mueller-Hinton agar plates containing 5% sheep blood, onto which the discs were placed and incubated overnight in an atmosphere of CO2. The size of the zone of inhibition was measured to determine the bacterium's susceptibility, which was interpreted as sensitive, intermediate, or resistant. Control strains were employed for quality assurance. Identification of antibiotic resistance genes and virulence: The lytA gene, encoding autolysin, was targeted to confirm pneumococcal virulence. Mutations in the folA gene contributed to resistance against trimethoprim-sulfamethoxazole (cotrimoxazole) by altering the dihydro- folate reductase enzyme, thereby reducing drug efficacy. The selected genes were amplified using conventional multiplex PCR, which enabled identification of several target genes in one reaction. The procedure involved the following steps: DNA extraction method: Boiling lysis (Quick Crude DNA Extraction) method based on the principle that heat disrupts the bacterial cell wall, releasing DNA.
The bacterial pellet was suspended in Tris-EDTA buffer.
The suspension was boiled at 95-100℃ for 10-15 minutes.
The DNA template (the supernatant) was used after cen- trifuge.
3) Primer selection
Specific primers for lytA (virulence gene) and folA (co- trimoxazole resistance gene) was used.
|
Gene |
Primer |
Size of amplicon (Base Pairs) |
|
lytA |
F: 5'-CAACCGTACAGAATGAAGCCG-3' |
319 bp |
|
R: 5'-TTATTCGTGCAATACTCGTGC-3' |
||
|
folA |
F: 5'-TGTAAGCTATTCCAAACCAG-3' |
600 bp |
|
R: 5'-CTACGTTCCATTAGACTTCC-3' |
|
Component |
Final
concentration |
Volume
(per 25 μl reaction) |
|
10X PCR Buffer |
1X |
2.5 μL |
|
dNTP mix (10 mM each) |
200 μM each |
0.5 μL |
|
Forward primer mix (10 μM each) |
0.2-0.5 μM each |
0.5-1.0 μL (adjust per target) |
|
Reverse primer mix (10 μM each) |
0.2-0.5 μM each |
0.5-1.0 μL (adjust per target) |
|
Taq DNA polymerase (5 U/μL) |
0.5-1.5 U |
0.2-0.5 μL |
|
Template DNA |
Variable (10-100 ng) |
1-5 μL |
|
MgCl2 (25 mM, if required) |
1.5-2.5 mM |
0.75-1.25 μL |
|
Nuclease-free water |
- |
Adjust to 25 μL total volume |
4) PCR amplification conditions
The PCR reaction mixture included primers, the template DNA, MgCl2, dNTPs, buffer, and Taq polymerase. Thermo- cycling conditions were optimized for the efficient amplifi- cation of both genes.
6. PCR procedure
In a PCR vial, 25 μL of Master Mix (Table 1) was combined with F and R primers, 1 μl each (sequence mentioned below; 10 pmoles/μl), genomic DNA (1 μl), and nuclease-free water (22 μl), yielding 50 μl total volume. The components were gently vortexed to ensure thorough mixing and briefly centri- fuged to collect any droplets. The vials were then placed into the PCR machine for amplification. The primer details are given in Table 2.
1) Conventional PCR method
The conventional PCR method was used. The conditions were set initially at 95℃ for 5 minutes for initial denaturation, then cycles (30) of amplification, at 94℃ × 60 seconds denaturing, 53℃ × 60 seconds primer annealing, and 72℃ × 30 seconds primer extension. The final step of PCR amplifi- cation involved raising the temperature to 72℃ × 7 minutes (final extension).
2) Positive and negative controls
(1) Positive control
ATCC Streptococcus pneumoniae 49619, which reliably expresses the target genes (e.g., lytA, folA), was included in each PCR run as a reference strain to validate reagent integrity and protocol performance.
(2) Negative control
A no-template control containing all PCR components except the DNA template was included to monitor contam- ination and nonspecific amplification.
3) Reagent and environmental monitoring
All PCR reagents were aliquoted to avoid repeated freeze-thaw cycles and stored under recommended conditions. DNA extraction and PCR setup were performed in separate dedicated areas using sterile, aerosol-resistant filter tips to prevent cross-contamination.
4) PCR replication
All clinical samples were tested in duplicate. Discrepant results were retested, and only concordant results were con- sidered for final analysis. For each batch, a minimum of 10% of samples were randomly selected for re-amplification to assess reproducibility and inter-assay variation.
5) Agarose gel electrophoresis procedure
A 2% agarose gel was prepared, and ethidium bromide (5 μL) was added at around 60℃ before casting. Once solid- ified, the gel was placed in a submarine tank filled with 1X Tris-Acetate-EDTA buffer. PCR products and a 100 bp DNA ladder were loaded, and electrophoresis was run at 50 V until dye migration was adequate. DNA bands were visualized using a UV transilluminator. The presence of specific bands corresponding to the lytA and folA genes indicate the suc- cessful amplification of the target sequences. Quality control was achieved using ATCC Streptococcus pneumoniae (49619).
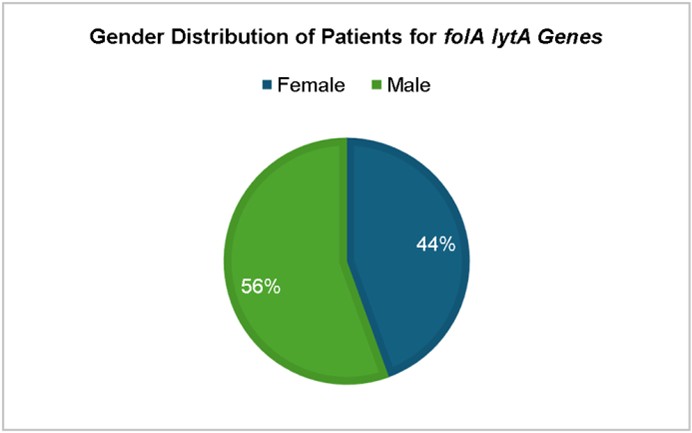
The study found that Streptococcus pneumoniae isolates exhibited characteristic morphology, with a gender distri- bution of female patients (60%). The age distribution of cases showed a higher prevalence in the 19-60 years age group (46%), with the highest proportion aged 19-40 years (22 cases). The prevalence of pneumococcal infections was highest in the elderly (4 cases). Sputum was the predominant sample (82%), indicating Streptococcus pneumoniae remains a leading respiratory pathogen, especially in hospitalized patients. Streptococcus pneumoniae isolates exhibited high resistance rates to tetracycline (66%) and erythromycin (60%), while remaining susceptible to cefotaxime, vancomycin, line- zolid, azithromycin, and cefazolin. The molecular detection of this gene further confirms the presence of drug resistance in clinical settings. lytA gene, essential for cell wall degradation, detected in 90% of isolates. folA gene, associated with cotrimoxazole resistance, also detected in 90% of isolates. Highest prevalence of folA and lytA in 46-60-year age group (34%). Higher prevalence of both genes in males (56%), suggesting possible host-related factors.
This study design was prospective and observational, and evaluated the clinical microbiological aspects of Streptococcus pneumoniae among individuals diagnosed with LRTIs. The 50 isolates in this study exhibited the characteristic morphology of Streptococcus pneumoniae, as observed microscopically and in culture.
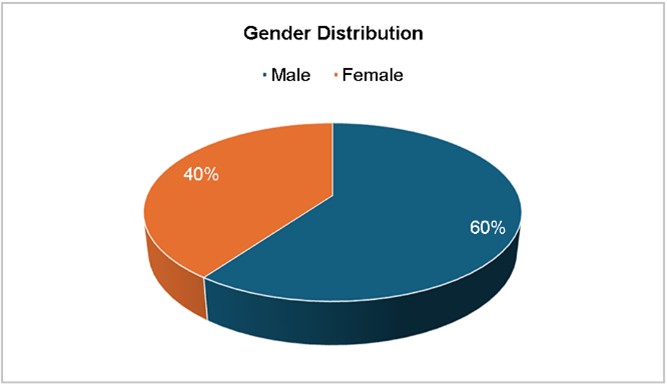
The gender distribution (Fig. 1) shows a predominance of isolates from female patients (60%), for a male-to-female ratio of roughly 1:2. This aligns with a previous study sug- gesting that while pneumococcal infections affect both genders, females may present more frequently in clinical settings due to increased healthcare-seeking behavior or other epidemiological factors9.
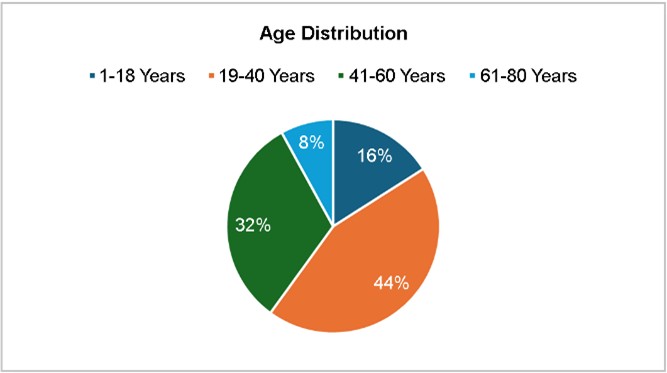
The age distribution of cases showed a higher prevalence in the 19-60 years age group, accounting for 46% of the cases, with the highest proportion aged 19-40 years (22 cases), followed by 41-60 years (16 cases). The lowest incidence was in the elderly (>60 years, 4 cases). The relatively lower prevalence in pediatric patients (1-18 years, 8 cases) could be attributed to effective pediatric vaccination programs against pneumococcal infections. However, the burden remains significant in adults, reinforcing the need for extended vac- cination strategies in older populations, as evidenced by Fig. 2. This age-related distribution aligns with that reported in a previous study indicating that adults, particularly those with underlying conditions, are more susceptible to pneumococcal infections10.
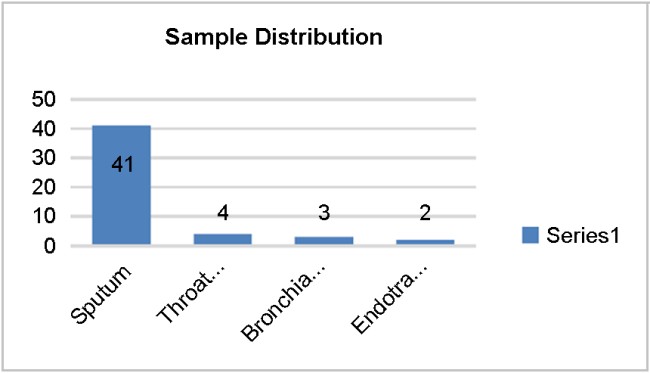
Among the different types of clinical samples analyzed, sputum was the predominant sample (82%), highlighting the respiratory tract as the primary site of pneumococcal infections. Other types of samples—throat swab (8%), bron- chial wash (6%), and endotracheal aspirates (4%)—were less frequently encountered (Fig. 3). This observation is consistent with the observed pathogenesis of respiratory infections, including CAP11. The high prevalence in sputum samples indicates that Streptococcus pneumoniae remains a leading respiratory pathogen, particularly in hospitalized patients.
In our study, Streptococcus pneumoniae isolates also ex- hibited high resistance rates to tetracycline (66%), and erythro- mycin (60%), while remaining susceptible to cefotaxime, vancomycin, linezolid, azithromycin, and cefazolin (Fig. 4). The antibiotic susceptibility testing revealed high sensitivity to cefotaxime, vancomycin, linezolid, azithromycin, and cefazolin (100%), suggesting that these antibiotics remain effective treatment options for pneumococcal infections. The emer- gence of erythromycin resistance is of concern, as macrolides are often used in treating pneumococcal pneumonia. Inter- mediate resistance patterns were observed for erythromycin (12%) and tetracycline (6%), indicating that a subset of isolates exhibited reduced susceptibility. Several studies have reported increasing resistance to cotrimoxazole, penicillin, and macrolides in pneumococcal isolates12. The increasing trend of antimicrobial resistance underscores the importance of judicious use of antibiotics and continuous surveillance to prevent the development of further resistance. For instance, one study in Ethiopia reported resistance rates of 42.5% for cotrimoxazole, 30% for tetracycline, and 32.5% for erythro- mycin, with no resistance observed for vancomycin and linezolid13. These comparisons highlight the global variability in antibiotic resistance patterns among S. pneumoniae isolates. Notably, our study indicates a significant increase in resistance rates compared to the 2020 Ethiopian study. Specifically, resistance to cotrimoxazole is more than double (42.5% to our 90%) as is tetracycline resistance (30% to our 66%), and erythromycin resistance is nearly double (32.5% to our 60%).
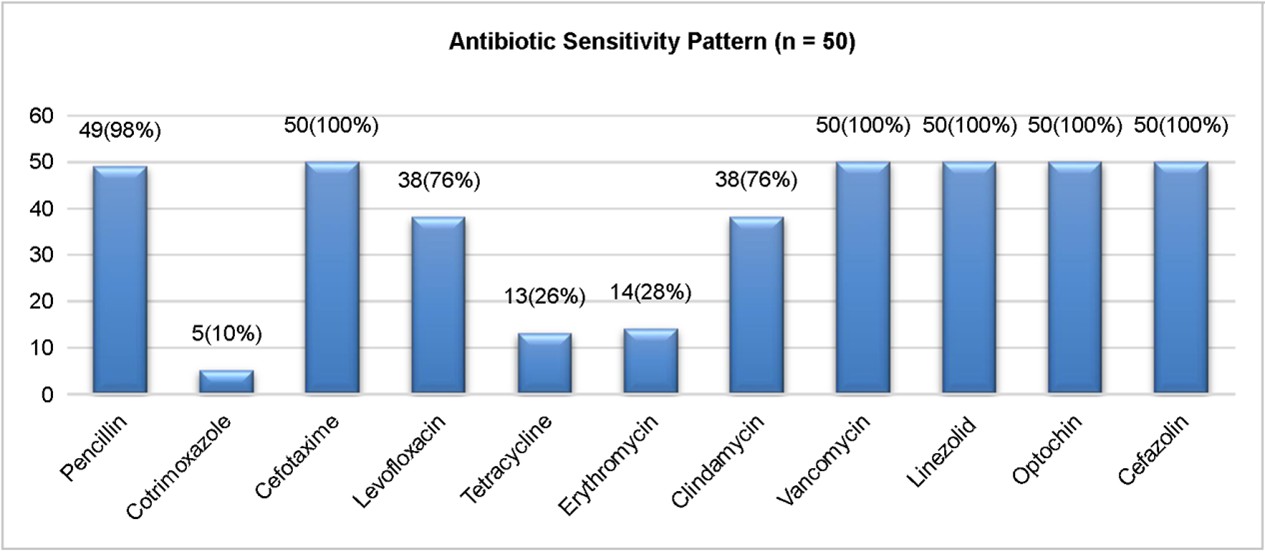
Antimicrobial resistance is a growing problem, particularly tertiary care settings where inappropriate antibiotic use con-tributes to resistance development14. In this study, 45 of the 50 isolates were resistant to cotrimoxazole (90%; Fig. 5). These rates of resistance observed in S. pneumoniae isolates are consistent with global trends, indicating an increasing burden of drug-resistant pneumococcal infections. Co- trimoxazole, an antibiotic commonly prescribed for respiratory infections, showed a significant decrease in efficiency in this study. Resistance to this drug is typically associated with mutations in the folA gene, which encodes dihydrofolate reductase, a key enzyme in folate biosynthesis15. The molecular detection of this gene further confirms the presence of drug resistance in clinical settings.
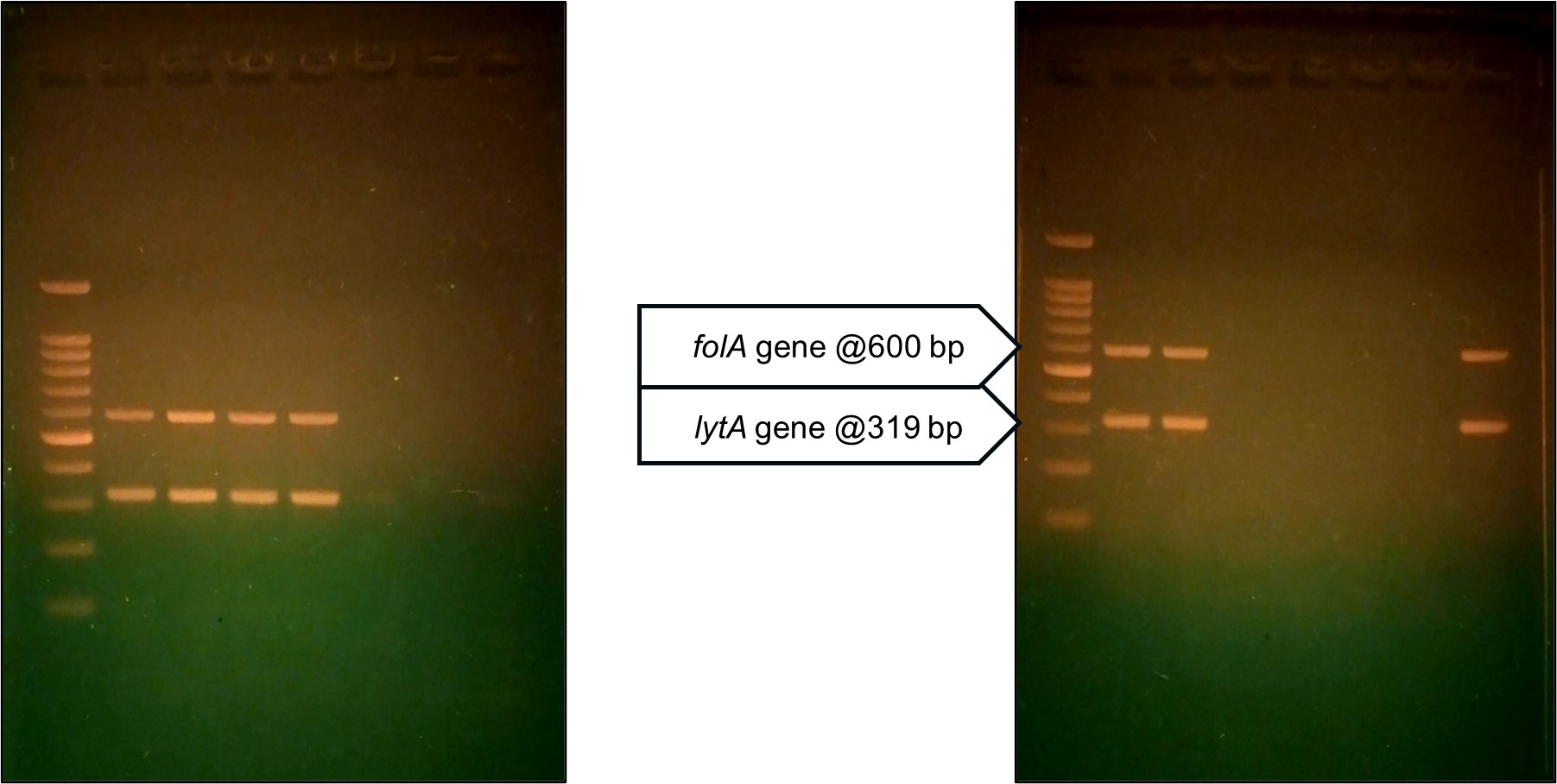
PCR analysis for the lytA and folA genes provided additional insights into pneumococcal pathogenesis and antimicrobial resistance. The lytA gene, which encodes autolysin and is essential for cell wall degradation, was detected in 45/50 (90%) isolates (Figs. 6 and 7). This high prevalence confirms the pneumococcal identity and its role in virulence. The folA gene, associated with resistance to cotrimoxazole, was also detected in 45/50 (90%) isolates, correlating with the observed resistance patterns.
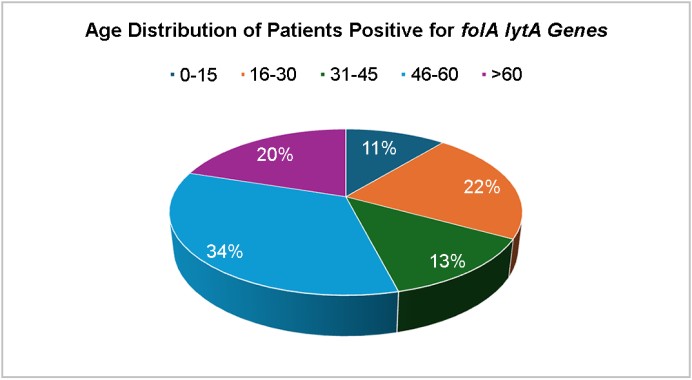
The highest prevalence of folA and lytA was observed in the 46-60-year age group (34%), followed by 16-30 years (22%). The gender distribution showed a higher prevalence of both genes in isolates from males (56%) than from females (44%) (Figs. 8 and 9), suggesting possible host-related factors influencing gene expression or acquisition16.
Optochin sensitivity and bile solubility are key phenotypic identification markers for Streptococcus pneumoniae. How- ever, the absence of the lytA gene, encoding the main autolysin, raises diagnostic concerns. Studies suggest that alternative autolysins like LytC and CbpD may mediate partial lysis in lytA-negative strains, preserving bile solubility. This highlights the need for molecular confirmation in atypical cases. According to Mosser et al., some lytA-negative pneumococcal strains may still undergo lysis via compensatory autolysins, maintaining bile solubility17.
One study has reported optochin sensitivity independent of lytA: Some Streptococcus pneumoniae strains lacking the lytA gene may still exhibit bile solubility due to alternative autolysins like LytC and CbpD. Optochin sensitivity remains unaffected, as it is mediated by ATPase inhibition and is independent of lytA. Certain Viridans group streptococci may mimic pneumococci phenotypically but lack virulence genes. Molecular assays targeting lytA, ply, and Spn9802, along with whole-genome sequencing, are essential for accurate identification and resistance tracking18.
This prospective observational study at Chettinad Hospital and Research Institute analyzed 50 isolates of drug-resistant Streptococcus pneumoniae obtained from a variety of respira- tory samples of LRTIs, with sputum the most common. A higher prevalence was noted among females aged 19-60 years. Molecular analysis revealed high resistance to cotrimoxazole, supported by the detection of the folA gene. The lytA gene served as a pneumococcal marker, although absent in some strains, suggesting possible alternative autolysin mechanisms. The study underscores rising resistance trends and advocates for molecular diagnostics alongside conventional methods. It calls for robust antibiotic stewardship and expanded pneumo- coccal vaccination programs. Whole-genome sequencing is recommended for tracking emerging resistance patterns. These findings stress the urgency of tailored therapies and sustained surveillance.
References
1. Ganaie F, Maruhn K, Li C, Porambo RJ, Elverdal PL, Abeygunwardana C, et al. Structural, genetic, and serological elucidation of Streptococcus pneumoniae serogroup 24 serotypes: discovery of a new serotype, 24C, with a variable capsule structure. J Clin Microbiol 2021;59:e0054021
Google Scholar
2. Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, Part I. Clin Infect Dis 2000;30:100-121
Google Scholar
3. Maeda H, Morimoto K. Global distribution and char- acteristics of pneumococcal serotypes in adults. Hum Vaccin Immunother 2025;21:2469424
Google Scholar
4. Schrag SJ, Beall B, Dowell SF. Limiting the resistant pneumococcal infections: the burden of disease and challenges in monitoring and controlling antimicrobial resistance. Clin Microbiol Rev 2000;13:588-601
Google Scholar
5. Klugman KP. Pneumococcal resistance to antibiotics. Clin Microbiol Rev 1990;3:171-196
Google Scholar
6. Kolhapure S, Yewale V, Agrawal A, Krishnappa P, Soumahoro L. Invasive pneumococcal disease burden and PCV coverage in children under five in Southeast Asia: implications for India. J Infect Dev Ctries 2021;15: 749-760
Google Scholar
7. Kumar P, Ray A, Kumari A, Sultana A, Hora R, Singh K, et al. Chroniclinga the journey of pneumococcal conjugate vaccine introduction in India. Vaccines 2025;13:432
8. Kulkarni N, Routray A, Taur S. A multicenter evaluation of overall susceptibility and antimicrobial resistance among Streptococcus pneumoniae isolates. Cureus 2023;15: e41984
Google Scholar
9. Gutiérrez F, Masiá M, Mirete C, Soldán B, Rodríguez JC, Padilla S, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect 2006;53:166-174
Google Scholar
10. Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol 2006;44:124-131
Google Scholar
11. Goyal R, Singh NP, Kaur M, Talwar V. Antimicrobial resistance in invasive and colonising Streptococcus pneumoniae in North India. Indian J Med Microbiol 2007; 25:256-259
Google Scholar
12. Weinberger DM, Malley R, Lipsitch M. Serotype replace- ment in disease after pneumococcal vaccination. Lancet 2011;378:1962-1973
Google Scholar
13. Sharew B, Moges F, Yismaw G, Abebe W, Fentaw S, Vestrheim D, et al. Antimicrobial resistance profile and multidrug resistance patterns of Streptococcus pneumo- niae isolates from patients suspected of pneumococcal infections in Ethiopia. Ann Clin Microbiol Antimicrob 2021;20:26
Google Scholar
14. Kamalova S. The antibiotic resistance in pneumonia. Mod Sci Res 2025;4:1384-1391
Google Scholar
15. Safari D, Putri HF, Bimantari A, Paramaiswari WT, Tafroji W, Khoeri MM, et al. Genetic characterization of co-trimoxazole non-susceptible Streptococcus pneumoniae isolates from Indonesia. Access Microbiol 2021;3:000271
Google Scholar
16. Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Raga-Luria X, Gomez-Bertomeu F. Epidemiology of community-acquired pneumonia in older adults: a population-based study. Respir Med 2009;103:309-316
Google Scholar
17. Mosser JL, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem 1970;245:287-298
Google Scholar
18. Simões AS, Tavares DA, Rolo D, Ardanuy C, Goossens H, Henriques-Normark B, et al. lytA-based identification methods can misidentify Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2016;85:141-148
Google Scholar
Congratulatory MessageClick here!