pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Son Thach Mao,Hue Van Thi Tran,Ngoc Hieu Nguyen
10.17966/JMI.2025.30.2.66 Epub 2025 July 01
Abstract
Edwardsiella tarda is a rare human causative bacterium, especially in extraintestinal infections. In this study, we described a case of E. tarda and Proteus vulgaris coinfection in a fish vendor who suffered a work-related injury during fish butchering. In this patient, E. tarda and P. vulgaris were resistant to several antibiotics, including ampicillin, colistin, trimethoprim-sulfamethoxazole, cefazolin, ceftriaxone, amikacin, cefepime, ciprofloxacin, ampicillin-sulbactam, and tigecycline. The patient was managed by necrotic tissue debridement, vacuum-assisted closure, skin grafting, and antibiotic treatment. After 2 weeks, the wound healed completely. To the best of our knowledge, this was the first case report of extraintestinal infection caused by multidrug-resistant E. tarda.
Keywords
Bocourti fish Edwardsiella tarda Extraintestinal infection Multidrug-resistant Proteus vulgaris
Edwardsiella tarda is a nonfermenting gram-negative bacillus belonging to the Enterobacteriaceae family. Although rarely associated with infections in humans1, E. tarda is a pathogen that is primarily responsible for gastroenteritis, typically from food-borne transmission. The colonization rate among humans ranges from 0.0073% in the Japanese population to 1% in Panamanians2,3. Reports on extraintes- tinal infections secondary to E. tarda are scarce, and the documented manifestations include endocarditis, hepato- biliary infection, empyema, peritonitis, intraabdominal abscess, wound infection, osteomyelitis, and meningitis4. The risk factors for extraintestinal infections are hepatobiliary diseases, malignancy, and diabetes mellitus4,5, and the routes of human transmission include intake of contaminated food, such as raw seafood; wound contamination in aquatic environments; and exposure to infected animals1. Majority of E. tarda isolates demonstrated uniform susceptibility to the following antibiotics effective against gram-negative bacteria: aminoglycosides, cephalosporins, β lactams, and fluoroquinolones5. Notably, trimethoprim/sulfamethoxazole-resistant E. tarda has been clinically isolated from a pediatric patient with X-linked chronic granulomatous disease and osteomyelitis in Japan6, but no multidrug-resistant strains of E. tarda were identified. However, several studies have indicated that at least 90% of E. tarda strains exhibit resistance to colistin7.
Proteus vulgaris is a gram-negative rod-shaped bacterium known for its ability to reduce nitrate, produce indole, and generate hydrogen sulfide, while also being catalase-positive8. This microorganism is commonly found in the intestinal tracts of humans and animals, as well as in the soil, water, and fecal matter. Infections associated with P. vulgaris can affect the urinary tract; wounds, including burn injuries; bloodstream; and the respiratory system. Moreover, P. vulgaris produces hemolysins that are cytotoxic to red blood cells, facilitating iron release, which promotes bacterial proliferation8.
In this case report, we presented the course of a fish vendor with multidrug-resistant E. tarda and P. vulgaris coinfection caused by bocourti fish handling and was successfully treated with antibiotics in combination with debridement, vacuum-assisted closure (VAC), and skin grafting.
A 56-year-old male fish vendor sustained a foot injury from the fin of a bocourti fish that he was butchering, causing superficial laceration measuring approximately 5~6 cm. He cleaned the wound himself with Betadine, wore protective boots, and continued working. After 2 days without treat- ment, he developed mild fever, and the wound became swollen, painful, and pus was discharging. He then came to us for medical consultation. He was diagnosed last year with diabetes mellitus based on an HbA1c of 14.3% and is being treated by an endocrinologist with long-acting insulin (20 IU), metformin (1,000 mg), and gliclazide (30 mg) once daily. He had a history of pulmonary tuberculosis, which he recovered from after completing the treatment regimen recommended by the Vietnamese Ministry of Health.
Physical examination revealed a 3 × 7-cm wound on the dorsum of the foot, with swelling, erythema, tenderness, and purulent discharge. We collected a sample for bacterial culture, isolation, and antibiotic susceptibility testing. Laboratory tests revealed elevated white blood cell count (16,450/mm3) with neutrophil predominance (12,220/mm3, 74.3%). C-reactive protein was elevated, and HbA1c was 8.56%. The patient was started on intravenous 1 g vancomycin and 2 g cefoxitin every 12 h. The same day, he underwent surgical debride- ment. Intraoperatively, necrosis of the subcutaneous tissue and extensor tendon sheath was observed. After excision of all necrotic tissues, the wound was irrigated with normal saline and 0.5% povidone-iodine. Glycemic control was managed by an endocrinologist using insulin mixtard 30/70 (38 IU) once daily. Moreover, the wound was dressed daily using standard gauze, and antibiotic therapy was continued.
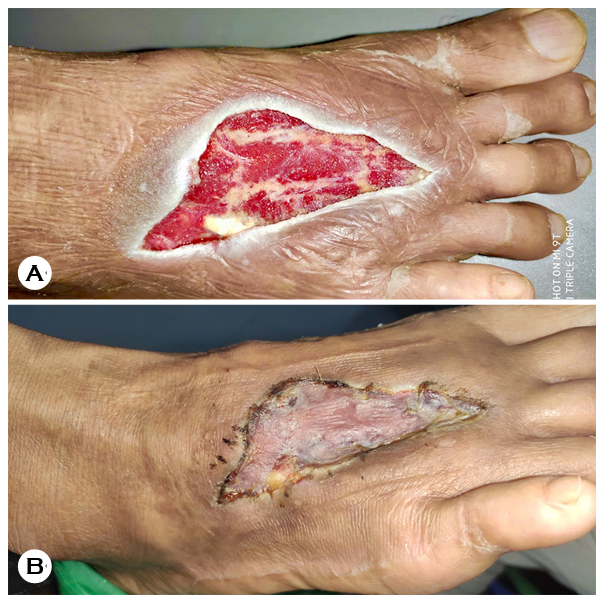
The culture results, which were based on colony morph- ology assessment and real-time PCR, indicated coinfection with E. tarda and P. vulgaris. Results of the antibiotic suscepti- bility assessment using the Epsilometer test are presented in Table 1. Accordingly, the antibiotic regimen was adjusted to intravenous 1 g ceftazidime every 8 h combined with 80 mg gentamicin every 12 h. After 10 days, gentamicin was discontinued, and VAC system was applied and changed every 3 days. After 7 days, the wound showed granulation tissue formation (Fig. 1A). The patient subsequently underwent a second surgery involving split-thickness skin grafting. Antibiotic therapy was maintained until complete wound healing, which was achieved within 2 weeks (Fig. 1B).
|
Antibiotic |
Edwardsiella |
Proteus |
|
Ampicilin |
R |
R |
|
Ceftazidime |
S |
S |
|
Colistin |
R |
R |
|
Meropenem |
S |
S |
|
Trimethoprim- |
R |
R |
|
Ampicillin-Sulbactam |
I |
S |
|
Ceftazidime-Avibactam |
S |
S |
|
Ertapenem |
S |
S |
|
Minocycline |
S |
R |
|
Cefazolin |
R |
R |
|
Ceftriaxone |
R |
R |
|
Gentamicin |
S |
S |
|
Piperacillin-Tazobactam |
S |
S |
|
Amikacin |
R |
- |
|
Cefepime |
R |
S |
|
Ciprofloxacin |
R |
R |
|
Imipenem |
S |
R |
|
Tigecycline |
I |
R |
|
S: sensitive, R: resistance, I: intermediate |
||
In humans, E. tarda is a rare pathogen primarily responsible for gastroenteritis as a food-borne infection1. Extraintestinal infections caused by E. tarda are reported infrequently, with sepsis being the most common manifestation (50%)5. More- over, E. tarda has been associated with various infections, such as cholecystitis, osteomyelitis, salpingitis, and peritonitis10. Soft tissue infections are typically acquired from aquatic injuries5. The risk factors of extraintestinal infections are hepatobiliary diseases, diabetes mellitus, and malignancy4.
This patient developed skin infection caused by injury during fish handling. This is a rare case of extraintestinal infection caused by E. tarda and can be considered an occupational accident, because the patient owns a fish facility. Hargreaves et al. previously reported a healthy patient who was stabbed by a catfish on the right arm and thigh; 12 h after, progressive erythema, edema, and limitation of motion of the right arm were observed, requiring emergency incision and drainage9. In our patient, a swollen, red, painful, purulent, and necrotic wound developed 2 days after the foot injury. A literature review indicated that 23.4% of E. tarda infections are skin and soft tissue infections (SSTIs), which were primarily from wounds associated with fresh and brackish water or expo- sure to aquatic organisms1. SSTI caused by E. tarda usually presents with erythema, warmth, pus, and pain and often leads to abscess formation5. In >50% of patients, E. tarda was reported to contribute to epithelial cell destruction and inflammation, leading to the progression of cellulitis to abscess10. In this patient, the wound continued to enlarge and failed to heal due to uncontrolled diabetes and coinfection of E. tarda and P. vulgaris. Proteus species, primarily P. vulgaris, is responsible for various infections, such as wound infections, food poisoning, and septicemia. A study in China found that approximately 48% of infected wounds harbored P. vulgaris11.
Patients with extraintestinal infections have been treated with antibiotics and/or surgical interventions, such as debride- ment and drainage4. The current study found no multidrug-resistant strains of E. tarda1. Although E. tarda may have chromosomally encoded β lactamases, it remains suscep- tible to almost all β lactam antibiotics, except penicillin and oxacillin12. According to Kohei Hasegawa et al., one strain of E. tarda exhibited resistance to both ampicillin and piperacillin, although susceptibility was restored by β lactam/β lactamase inhibitors12. All cephalosporins were effective, indicating that the β lactamase produced by E. tarda is probably a peni- cillinase. Several studies have shown that at least 90% of E. tarda strains are resistant to colistin7. In this patient, both E. tarda and P. vulgaris strains were resistant to most antibiotics, including ampicillin, colistin, trimethoprim-sulfamethoxazole, cefazolin, ceftriaxone, amikacin, cefepime, ciprofloxacin, ampicillin-sulbactam, and tigecycline. In a multicenter study, antibiotic susceptibility tests indicated that P. vulgaris exhibited resistance to sulfonamides13.
In all three regions of Vietnam, the use of antibiotics and other antimicrobial chemicals is common in fish and shrimp farming, with more than 20 types of antibiotics already used, including those on the banned list14. In recent years, overuse of antibiotics for disease prevention and treatment has posed several major challenges to the aquaculture industry and human health globally15. Antibiotic-resistant bacteria can grow and spread between aquatic animals and humans through direct contact or the food chain and environment and cause serious life-threatening infections16. Moreover, E. tarda isolated from farmed fish sometimes carries plasmids associated with drug resistance17. Therefore, E. tarda and P. vulgaris can easily develop multidrug resistance mutations, especially in the current conditions of seafood farming in Vietnam. In this case, we believe that the bocourti fish was infected with multidrug-resistant E. tarda and P. vulgaris from their farming environment and infected the fish vendor through the wound.
After obtaining paraclinical tests and bacterial cultures in this patient, we performed necrotic tissue debridement com- bined with VAC, skin grafting, and antibiotic treatment. VAC has been widely used worldwide, including in Vietnam, for treating incision infections and has yielded excellent results. It removes secretions, reduces bacterial infiltration and edema to improve local perfusion flow, and provides oxygen and nutrients necessary for wound recovery, granular tissue pro- liferation, and microvascular neoplasia18. Abscess formation is frequently observed in E. tarda infections. In a small single-center study on five patients with skin infection, surgical drainage was required in all patients, myonecrosis developed in one, and bacteremia was recorded in one5.
Notably, the antibiotics administered to this patient were effective, despite the fact that E. tarda is resistant to most antibiotics. In the past, E. tarda was thought to contain genes encoding β lactamase12; this explains the high likelihood of resistance to β lactam antibiotics. In addition, Waltman et al. suggested that E. tarda likely produces penicillinase, based on findings that cephalosporin treatment remained effective7. In this patient, we used the antibiogram results as basis for our decision to use ceftazidime, a third-generation cephalo- sporin, along with gentamicin. We used gentamicin for the first 10 days, because it helped hasten infection control and reduced initial bacterial load. After the infection was controlled, gentamicin was discontinued to reduce the risk of nephro- toxicity19.
In this patient, surgical debridement played a crucial role. Debridement is necessary to remove necrotic tissue and thoroughly clean soft tissues, and antibiotic therapy is essential to eradicate the bacterial pathogens. Therefore, surgical management and antibiotic therapy are equally important and complementary and must be combined to achieve successful treatment outcomes.
We presented the first reported case of wound infection caused by E. tarda following a bocourti fish fin injury, which was successfully treated with antimicrobials, surgery, and VAC. Although a rare pathogen in humans, E. tarda can be fatal. In particular, special attention should be given to the multidrug-resistant strains of E. tarda to ensure effective treatment for patients.
References
1. Hirai Y, Asahata-Tago S, Ainoda Y, Fujita T, Kikuchi K. Edwardsiella tarda bacteremia. A rare but fatal water-and foodborne infection: review of the literature and clinical cases from a single centre. Can J Infect Dis Med Microbiol 2015;26:313-318
Google Scholar
2. Onogawa T, Terayama T, Zenyoji H, Amano Y, Suzuki K. Distribution of Edwardsiella tarda and hydrogen sulfide-producing Escherichia coli in healthy persons. Kansenshogaku Zasshi 1976;50:10-17
Google Scholar
3. Kourany M, Vasquez MA, Saenz R. Edwardsiellosis in man and animals in Panamá: clinical and epidemiological characteristics. Am J Trop Med Hyg 1977;26:1183-1190
Google Scholar
4. Wang IK, Kuo HL, Chen YM, Lin CL, Chang HY, Chuang FR, et al. Extraintestinal manifestations of Edwardsiella tarda infection. Int J Clin Pract 2005;59:917-921
Google Scholar
5. Slaven EM, Lopez FA, Hart SM, Sanders CV. Myonecrosis caused by Edwardsiella tarda: a case report and case series of extraintestinal E. tarda infections. Clin Infect Dis 2001;32:1430-1433
Google Scholar
6. Kawai T, Kusakabe H, Seki A, Kobayashi S, Onodera M. Osteomyelitis due to trimethoprim/sulfamethoxazole-resistant Edwardsiella tarda infection in a patient with X-linked chronic granulomatous disease. Infection 2011; 39:171-173
Google Scholar
7. Waltman WD, Shotts EB. Antimicrobial susceptibility of Edwardsiella tarda from the United States and Taiwan. Vet Microbiol 1986;12:277-282
Google Scholar
8. Kim BN, Kim NJ, Kim MN, Kim YS, Woo JH, Ryu J. Bacteraemia due to tribe Proteeae: a review of 132 cases during a decade (1991-2000). Scand J Infect Dis 2003; 35:98-103
Google Scholar
9. Hargreaves JE, Lucey DR. Life-threatening Edwardsiella tarda soft-tissue infection associated with catfish puncture wound. J Infect Dis 1990;162:1416-1417
Google Scholar
10. Janda JM, Abbott SL, Kroske-Bystrom S, Cheung WK, Powers C, Kokka RP, et al. Pathogenic properties of Edwardsiella species. J Clin Microbiol 1991;29:1997-2001
Google Scholar
11. Feng Y, Yang Z, Li D, Li J, Li D, de Hoog S, et al. Nails and skin co-infection by Fusarium verticillioides and Proteus vulgaris secondary to arterial occlusion of lower extremity. Rev Iberoam Micol 2024;41:37-42
Google Scholar
12. Hasegawa K, Kenya M, Suzuki K, Ogawa Y. Character- istics and prognosis of patients with Edwardsiella tarda bacteremia at a single institution, Japan, 2005-2022. Ann Clin Microbiol Antimicrob 2022;21:56
Google Scholar
13. Ma Lin ML, Yan Li YL, Han LiHua HL, Zhou ChunJu ZC. Cutaneous Fusarium moniliforme infection in a child. J Clin Dermatol 2006;35:532-534
14. Cường TK, Hà PTH, Luân NT. Research for the potential of some plant extracts in the prevention of many anti- biotic resistance bacteria that cause disease on climbing perch (Anabas testudineus). Ho Chi Minh City Open University Journal of Science 2023;2:74-88
15. Hossain A, Habibullah-Al-Mamun M, Nagano I, Masunaga S, Kitazawa D, Matsuda H. Antibiotics, antibiotic-resistant bacteria, and resistance genes in aquaculture: risks, current concern, and future thinking. Environ Sci Pollut Res Int 2022;29:11054-11075
Google Scholar
16. Okeke ES, Chukwudozie KI, Nyaruaba R, Ita RE, Oladipo A, Ejeromedoghene O, et al. Antibiotic resistance in aquaculture and aquatic organisms: a review of current nanotechnology applications for sustainable manage- ment. Environ Sci Pollut Res Int 2022;29:69241-69274
Google Scholar
17. Preena PG, Dharmaratnam A, Raj NS, Raja SA, Nair RR, Swaminathan TR. Antibiotic-resistant Enterobacteriaceae from diseased freshwater goldfish. Arch Microbiol 2021; 203:219-231
Google Scholar
18. Shalaby M, Emile S, Elfeki H, Sakr A, Wexner SD, Sileri P. Systematic review of endoluminal vacuum-assisted therapy as salvage treatment for rectal anastomotic leakage. BJS Open 2018;3:153-160
Google Scholar
19. Prins JM, Buller HR, Kuijper EJ, Tange RA, Speelman P. Once versus thrice daily gentamicin in patients with serious infections. Lancet 1993;341:335-339
Google Scholar
Congratulatory MessageClick here!