pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Yong Hyun Jang,Nam Gyoung Ha,Ho-Sung Ryu,Chanik Park,Dong Soo Lee,Jin Hyuck Kim,Weon Ju Lee
10.17966/JMI.2025.30.2.58 Epub 2025 July 01
Abstract
Background: Aromatherapy using natural essential oils has been proposed as a complementary strategy to prevent respiratory infections, particularly in elderly populations vulnerable to microbial colonization and antibiotic resistance.
Objective: To evaluate the effect of inhaling the cypress essential oil complex on the nasal microbiome.
Methods: A clinical study was conducted with 14 participants (mean age: 73 years) who were randomly assigned to either a treatment group (n = 8) or placebo group (n = 6). The treatment group was exposed to a natural essential oil complex containing cypress, lavender, eucalyptus, pine needle, rosemary, German chamomile, and bergamot oils for 3 hours daily over 4 weeks. The placebo group received a 1/100 dilution of the formulation. Nasal swabs were collected before and after treatment and were analyzed for changes in microbial composition. Statistical significance was assessed using the paired t-test.
Results: The treatment group exhibited a significant reduction in the genus Staphylococcus (1.92% to 0.62%, p < 0.05) and a decrease in Streptococcus pneumoniae (1.04% to 0.87%). In contrast, the placebo group experienced minimal or no adverse changes. No adverse effects were reported during the study period.
Conclusion: Inhalation of the cypress essential oil complex was associated with a reduction in nasal colonization by potential respiratory pathogens in older adults. These preliminary findings suggest a possible microbiome-modulating effect that warrants further investigation in larger, outcome-based clinical studies.
Keywords
Aged Aromatherapy Microbiota Respiratory tract infection Staphylococcus aureus
Infections of the respiratory tract remain a major cause of morbidity and mortality in the elderly population, primarily due to age-related immunosenescence and a higher prevalence of chronic comorbidities1. Among these infections, pneumonia is particularly prevalent and frequently associated with hospital- ization and adverse outcomes2. The nasal cavity, as a primary entry site for airborne pathogens, plays a pivotal role in the pathogenesis of lower respiratory tract infections3. In healthy adults, the microbiota of the nasal cavity is predominantly composed of commensal genera such as Corynebacterium, Staphylococcus, and Propionibacterium, which play key roles in maintaining mucosal homeostasis and suppressing the overgrowth of pathogenic organisms4. Colonization of the nasal mucosa by pathogenic bacteria, notably Staphylococcus aureus and Streptococcus pneumoniae, is a known risk factor for respiratory infections5,6. The composition of the nasal microbiome has thus emerged as a relevant factor that influences an individual's susceptibility to infection7. Modulating the nasal microbial environment to suppress colonization by respiratory pathogens may represent a viable strategy for infection prevention, particularly in older adults8.
Amid growing concerns regarding antibiotic resistance and the adverse effects of prolonged antimicrobial use, there is increasing interest in complementary nonpharmaceutical approaches to infection control9. Due to its antimicrobial, anti-inflammatory, and mucolytic properties, aromatherapy using natural essential oils has gained attention10. Essential oils contain volatile compounds such as monoterpenes and phenolics, many of which have demonstrated broad-spectrum antibacterial activity in vitro10. Cypress essential oil (Cupressus sempervirens), which is traditionally used in Mediterranean medicine, possesses antiseptic and respiratory-soothing effects and has demonstrated antimicrobial activity against respiratory pathogens under experimental conditions11. Although clinical data in humans remain limited, its therapeutic potential is supported by pharmacologic evidence and documented synergistic effects with other essential oils such as lavender, eucalyptus, rosemary, and chamomile12.
This study aimed to evaluate the effects of inhaling a cypress-based essential oil complex inhalation on the nasal micro- biome in older adults, with a particular focus on its ability to reduce colonization by pathogenic bacteria such as S. aureus and S. pneumoniae.
1. Preparation of the essential oil complex
The test formulation, referred to as the Cypress Essen- tial Oil Complex No. 8, was prepared by blending seven natural essential oils with known antimicrobial properties. The essential oil composition was as follows: cypress essential oil (C. sempervirens, 50 g), lavender essential oil (Lavandula angustifolia, 20 g), eucalyptus essential oil (Eucalyptus glo- bulus, 10 g), pine needle essential oil (Pinus sylvestris, 10 g), rosemary essential oil (Rosmarinus officinalis, 5 g), German chamomile essential oil (Matricaria chamomilla, 3 g), and bergamot essential oil (Citrus bergamia, 2 g). All essential oils were purchased from a certified commercial supplier and blended in a sterile facility to ensure consistency and purity.
2. Study design and participants
All participants were community-dwelling older adults with no clinical signs of upper respiratory tract infection at the time of enrollment. The final cohort consisted of 14 participants (mean age: 73.4 ± 10.3 years; range: 55~89 years), including 5 men and 9 women. All participants were in generally stable health. Documented chronic conditions included 2 participants with hypertension, 2 with type 2 diabetes, 1 with hyperlipidemia, and 1 with both hypertension and hyper- lipidemia. No participants had clinical evidence of immuno- suppression, recent antibiotic use, or active respiratory illness at enrollment. We randomly assigned participants to either a treatment group (n = 8) or a placebo group (n = 6). The treatment group was exposed to the cypress essential oil complex for 3 hours daily over a 4-week period, whereas the placebo group received an aromatherapy product diluted to 1/100th the concentration of the active formulation.
3. Application protocol
Each participant received two identical glass containers filled with either the active formulation or placebo. They were instructed to place the containers in the room where they spent the most time (typically bedroom or living room) and to ensure passive inhalation exposure for at least 3 hours daily for 4 consecutive weeks. To maintain consistent aromatic exposure throughout the study period, the containers were replaced when the remaining liquid level fell below 0.5 cm.
4. Microbial analysis
We collected nasal swabs from all participants before and after the 4-week intervention period. We obtained samples from the anterior nares by gently rotating a sterile swab approximately 1 to 2 cm into each nostril, following standard nasal screening protocols commonly used for microbial car- riage studies. We extracted genomic DNA using a QIAamp DNA Stool Mini Kit (QIAGEN), and the V4 region of the 16S rRNA gene was amplified for microbial profiling. Sequen- cing was performed on the Illumina iSeq 100 platform, and taxonomic classification was conducted using the EZBioCloud pipeline and Illumina BaseSpace. The raw sequencing gener- ated approximately 100,000 reads per sample. After quality control and filtering, the average read count for downstream analysis was approximately 42,831 (range: 26,868~55,982). All samples passed the quality thresholds and were included in the final analysis. Although relative abundance at the genus level was reliably assessed, the interpretation at the species level (e.g., S. aureus, S. pneumoniae) should be made with caution due to the inherent resolution limits of 16S rRNA analysis.
5. Statistical analysis
The primary outcome was the change in the relative abundance of target bacterial genera and species pre and postintervention. Within-group differences were evaluated using paired t-tests, and between-group comparisons were conducted using independent sample t-tests. We considered a p value of <0.05 to be statistically significant.
6. Ethical considerations
The Institutional Review Board of Kyungpook National University Hospital reviewed and approved the study protocol (IRB No. KNUH 2019-06-007-001). All participants provided written informed consent before enrollment in accordance with the Declaration of Helsinki.
1. Reduction of nasal microbes at the genus level
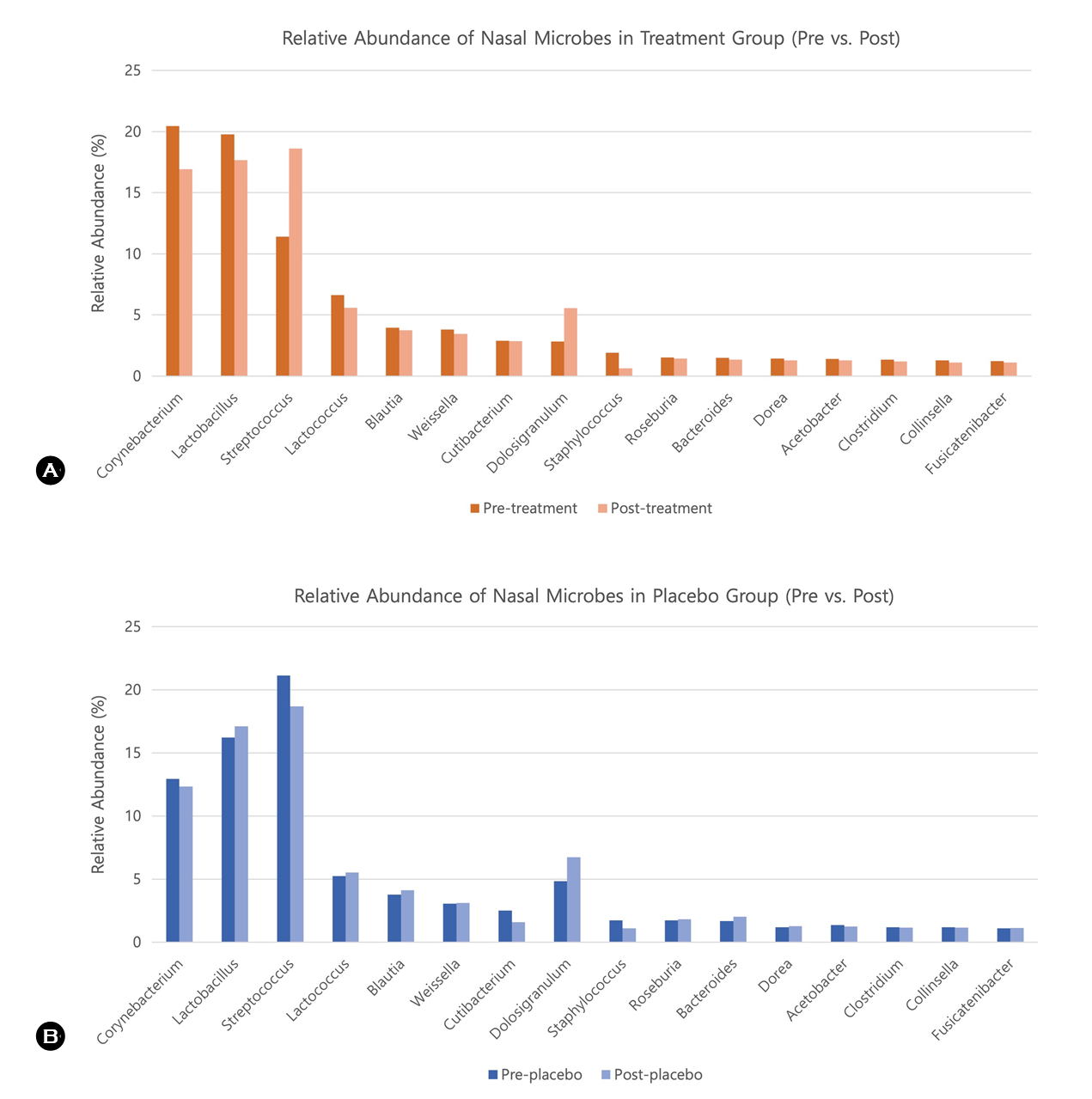
As shown in Table 1 and Fig. 1, the treatment group exhibited a notable reduction in the relative abundance of potentially pathogenic bacterial genera after 4 weeks of essen- tial oil exposure. The genus Staphylococcus decreased from 1.92% at baseline to 0.62% postintervention in the treat- ment group, whereas the placebo group showed a smaller reduction from 1.74% to 1.11%. For the genus Clostridium, the treatment group experienced a slight decrease from 1.33% to 1.19%, whereas the placebo group experienced a minimal change from 1.18% to 1.16%. The bar chart in Fig. 1 visually illustrates these pre and postintervention shifts in microbial composition at the genus level.
|
Genus |
Treatment group |
|
Placebo group |
||
|
Pre-treatment |
Post-treatment |
Pre-placebo |
Post-placebo |
||
|
Corynebacterium |
20.47 |
16.91 |
|
12.94 |
12.33 |
|
Lactobacillus |
19.76 |
17.68 |
|
16.22 |
17.10 |
|
Streptococcus |
11.40 |
18.61 |
|
21.13 |
18.68 |
|
Lactococcus |
6.64 |
5.60 |
|
5.23 |
5.54 |
|
Blautia |
3.96 |
3.74 |
|
3.78 |
4.11 |
|
Weissella |
3.81 |
3.46 |
|
3.07 |
3.11 |
|
Cutibacterium |
2.90 |
2.86 |
|
2.52 |
1.58 |
|
Dolosigranulum |
2.82 |
5.55 |
|
4.83 |
6.74 |
|
Staphylococcus |
1.92 |
0.62 |
|
1.74 |
1.11 |
|
Roseburia |
1.52 |
1.44 |
|
1.75 |
1.81 |
|
Bacteroides |
1.48 |
1.34 |
|
1.67 |
2.02 |
|
Dorea |
1.42 |
1.27 |
|
1.20 |
1.29 |
|
Acetobacter |
1.39 |
1.28 |
|
1.37 |
1.24 |
|
Clostridium |
1.33 |
1.19 |
|
1.18 |
1.16 |
|
Collinsella |
1.27 |
1.11 |
|
1.19 |
1.16 |
|
Fusicatenibacter |
1.22 |
1.10 |
|
1.10 |
1.13 |
2. Reduction in nasal microbes at the species level
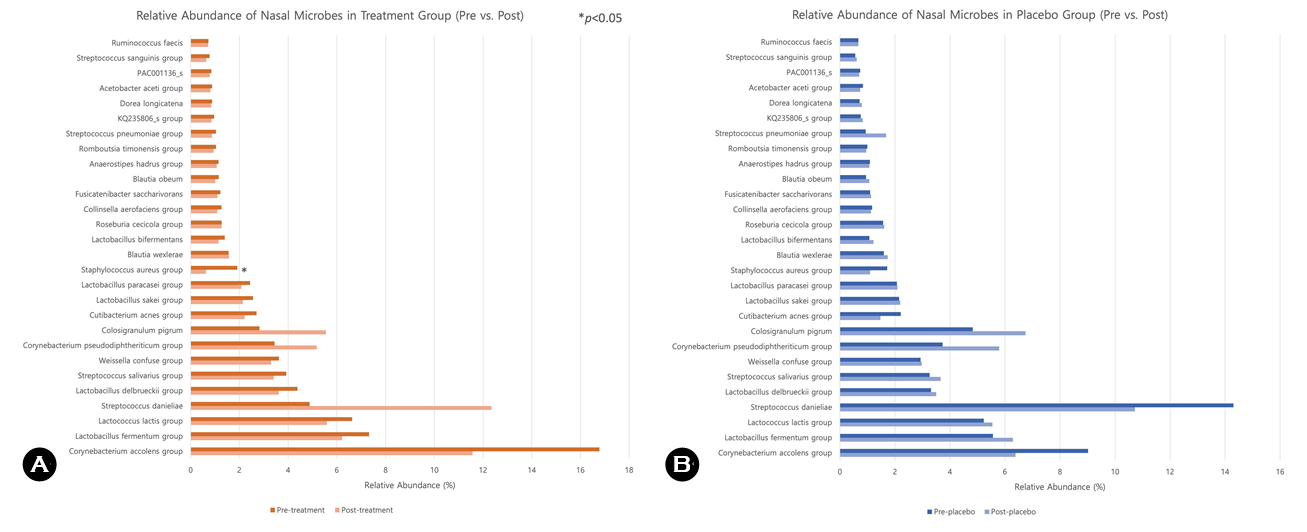
As presented in Table 2 and Fig. 2, there was a statistically significant decrease in S. aureus in the treatment group (p < 0.05), whereas we observed no meaningful reduction in the placebo group. In addition, the relative abundance of S. pneumoniae decreased from 1.04% to 0.87% in the treat- ment group. Conversely, the placebo group exhibited an increase in S. pneumoniae from 0.94% to 1.68%. Fig. 2 provides a visual comparison of these changes at the species level, highlighting the differential microbial responses between groups. No adverse events were reported in either group during the study period.
|
Species |
Treatment group |
|
Placebo group |
||
|
Pre- |
Post- |
Pre- |
Post- |
||
|
Corynebacterium
accolens group |
16.78 |
11.57 |
|
9.02 |
6.38 |
|
Lactobacillus
fermentum group |
7.32 |
6.21 |
|
5.56 |
6.29 |
|
Lactococcus
lactis group |
6.63 |
5.59 |
|
5.23 |
5.54 |
|
Streptococcus
danieliae |
4.88 |
12.35 |
|
14.30 |
10.72 |
|
Lactobacillus
delbrueckii group |
4.38 |
3.61 |
|
3.31 |
3.50 |
|
Streptococcus
salivarius group |
3.92 |
3.40 |
|
3.26 |
3.66 |
|
Weissella
confuse group |
3.62 |
3.30 |
|
2.93 |
2.97 |
|
Corynebacterium
pseudodiphtheriticum group |
3.44 |
5.17 |
|
3.73 |
5.79 |
|
Colosigranulum
pigrum |
2.82 |
5.55 |
|
4.83 |
6.74 |
|
Cutibacterium
acnes group |
2.70 |
2.21 |
|
2.21 |
1.47 |
|
Lactobacillus
sakei group |
2.56 |
2.14 |
|
2.15 |
2.19 |
|
Lactobacillus
paracasei group |
2.44 |
2.08 |
|
2.07 |
2.09 |
|
Staphylococcus
aureus group* |
1.91 |
0.62 |
|
1.72 |
1.10 |
|
Blautia
wexlerae |
1.56 |
1.57 |
|
1.60 |
1.74 |
|
Lactobacillus
bifermentans |
1.40 |
1.14 |
|
1.07 |
1.22 |
|
Roseburia
cecicola group |
1.27 |
1.27 |
|
1.57 |
1.61 |
|
Collinsella
aerofaciens group |
1.26 |
1.09 |
|
1.17 |
1.13 |
|
Fusicatenibacter
saccharivorans |
1.22 |
1.10 |
|
1.10 |
1.13 |
|
Blautia
obeum |
1.15 |
1.01 |
|
0.95 |
1.07 |
|
Anaerostipes
hadrus group |
1.14 |
1.07 |
|
1.09 |
1.07 |
|
Romboutsia
timonensis group |
1.04 |
0.94 |
|
1.00 |
0.96 |
|
Streptococcus
pneumoniae group |
1.04 |
0.87 |
|
0.94 |
1.68 |
|
KQ235806_s group |
0.96 |
0.85 |
|
0.76 |
0.83 |
|
Dorea longicatena |
0.88 |
0.84 |
|
0.72 |
0.80 |
|
Acetobacter aceti group |
0.88 |
0.81 |
|
0.84 |
0.74 |
|
PAC001136_s |
0.85 |
0.78 |
|
0.74 |
0.70 |
|
Streptococcus sanguinis group |
0.77 |
0.64 |
|
0.56 |
0.61 |
|
Ruminococcus faecis |
0.73 |
0.71 |
|
0.67 |
0.67 |
|
*p < 0.05 |
|||||
In this study, we demonstrated that the inhalation of a cypress-based essential oil complex was associated with a measurable reduction in the relative abundance of nasal pathogens, particularly S. aureus and S. pneumoniae, among elderly participants. These findings suggest that essential oil–based aromatherapy is a safe and noninvasive approach to modulating the nasal microbiome in populations at high risk of respiratory infections. The antimicrobial effects of essential oils are thought to involve multiple mechanisms, including disruption of microbial cell membranes, increased permeability, and leakage of intracellular contents13. Certain components, such as thymol and carvacrol, may also interfere with quorum sensing and inhibit the formation of biofilm13. These actions likely contribute to the observed reduction in nasal colon- ization by S. aureus and S. pneumoniae following essential oil inhalation.
The antimicrobial activity observed in this study is consistent with previous in vitro research highlighting the efficacy of cypress essential oil (C. sempervirens) against common respiratory pathogens14. The essential oil blend used in the present study contained not only cypress oil but also other botanicals such as lavender, eucalyptus, rosemary, and chamo- mile, which are known to exert complementary or synergistic antimicrobial effects12. These combinations may enhance the permeability of the membrane or the stability of active compounds, potentially contributing to the overall reduction in bacterial load.
Of particular interest is the significant reduction in S. aureus incidence in the treatment group. Nasal colonization by S. aureus is a well-established risk factor for subsequent respira- tory tract infections, and decolonization strategies have been explored primarily in hospital and long-term care settings15. The findings from this study support the potential use of aromatherapy as a community-based adjunct for reducing nasal carriage of this organism, particularly in older adults who may be more susceptible to infections and complications.
No adverse effects were reported throughout the inter- vention, further supporting the safety profile of the essential oil complex when used in a passive inhalation format. The simplicity of administration and low risk of systemic toxicity render aromatherapy an attractive candidate for integration into nonpharmacologic preventive strategies, especially in vulnerable elderly populations.
Nonetheless, we must acknowledge several limitations of this study. The small sample size reduced the statistical power and limited the generalizability of our findings. The 4-week duration might not have been sufficient to assess the long-term microbiome stability or clinical outcomes. Although we collected demographic and health information, the study design could not fully control for potential confounders such as environmental exposure, hygiene habits, and individual immune status. Because of the lack of individual-level micro- bial data, between-group statistical comparisons were not feasible; as such, our interpretations are limited to within-group trends. Furthermore, nonparametric methods and multiple comparison adjustments (e.g., false discovery rate correction) were not applied. Because the changes in relative abundance were modest—often less than 1%—caution is warranted when interpreting their clinical relevance. Future studies with larger cohorts, extended follow-up, and improved analytical approaches are needed to confirm these preliminary findings.
In conclusion, this preliminary study suggests that the inhalation of a cypress-based essential oil complex may be associated with a reduction in nasal colonization associated with potentially pathogenic bacteria in elderly individuals. Although the findings highlight a possible microbiome-modulating effect, further large-scale studies are needed to confirm its clinical relevance and potential role in supporting respiratory health.
References
1. Murray MA, Chotirmall SH. The impact of immuno- senescence on pulmonary disease. Mediators Inflamm 2015;2015:692546
Google Scholar
2. Torres A, Cilloniz C, Niederman MS, Menendez R, Chalmers JD, Wunderink RG, et al. Pneumonia. Nat Rev Dis Primers 2021;7:25
3. Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to re- spiratory health. Nat Rev Microbiol 2017;15:259-270
Google Scholar
4. Bassis CM, Tang AL, Young VB, Pynnonen MA. The nasal cavity microbiota of healthy adults. Microbiome 2014;2: 27
Google Scholar
5. Sakr A, Bregeon F, Mege JL, Rolain JM, Blin O. Staphylo- coccus aureus nasal colonization: an update on mechan- isms, epidemiology, risk factors, and subsequent infections. Front Microbiol 2018;9:2419
Google Scholar
6. Urban BC, Goncalves ANA, Loukov D, Passos FM, Reine J, Gonzalez-Dias P, et al. Inflammation of the nasal mucosa is associated with susceptibility to experimental pneumo- coccal challenge in older adults. Mucosal Immunol 2024; 17:973-989
Google Scholar
7. Flynn M, Lyall Z, Shepherd G, Lee ONY, Marianna Da Fonseca I, Dong Y, et al. Interactions of the bacteriome, virome, and immune system in the nose. FEMS Microbes 2022;3:xtac020
Google Scholar
8. Dimitri-Pinheiro S, Soares R, Barata P. The Microbiome of the nose-friend or foe? Allergy Rhinol (Providence) 2020;11:2152656720911605
Google Scholar
9. Hader A, Kose-Vogel N, Schulz L, Mlynska L, Hornung F, Hagel S, et al. Respiratory infections in the aging lung: implications for diagnosis, therapy, and prevention. Aging Dis 2023;14:1091-1104
Google Scholar
10. Horvath G, Acs K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: a review. Flavour Fragr J 2015;30:331-341
Google Scholar
11. Batiha GE, Teibo JO, Shaheen HM, Akinfe OA, Awad AA, Teibo TKA, et al. Bioactive compounds, pharmacological actions and pharmacokinetics of Cupressus sempervirens. Naunyn Schmiedebergs Arch Pharmacol 2023;396:389-403
Google Scholar
12. Orchard A, van Vuuren S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid Based Complement Alternat Med 2017;2017:4517971
Google Scholar
13. Chouhan S, Sharma K, Guleria S. Antimicrobial activity of some essential oils – present status and future per- spectives. Medicines (Basel) 2017;4:58
Google Scholar
14. Leigh-de Rapper S, Viljoen A, van Vuuren S. Essential oil blends: the potential of combined use for respiratory tract infections. Antibiotics (Basel) 2021;10:1517
Google Scholar
15. Piewngam P, Otto M. Staphylococcus aureus coloni- sation and strategies for decolonisation. Lancet Microbe 2024;5:e606-e618
Google Scholar
Congratulatory MessageClick here!