pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Kpongbo Etienne Angora,Maurine Aline N'guiachi,Matenin Ouattara,Arlette N'dri,Vincent Djohan,William Yavo,Hervé Eby Menan,Adèle Kacou N'douba
10.17966/JMI.2025.30.2.49 Epub 2025 July 01
Abstract
Background: Reusable surgical equipment can be a source of healthcare-associated infections. In Côte d'Ivoire, West Africa, data on fungal contamination of small surgical instruments are limited.
Objective: To assess fungal carriage in small surgical instruments at a university hospital in Angré, Côte d'Ivoire.
Methods: This cross-sectional study was conducted using small surgical instruments in six departments of a tertiary university hospital in Angré from February to July 2024. Samples were collected by swabbing instruments before sterilization and after opening random sterile sets. Direct examination and culture on Sabouraud dextrose agar medium containing chloramphenicol were performed. Fungal spores and hyphae were identified macroscopically and microscopically, whereas yeasts were identified using chromogenic medium and VITEK 2.
Results: Of 313 samples, 70 yielded positive culture (22.4%). Fungi on surgical instruments were identified most frequently at the ear, nose and throat service (34.8%), followed by the dental service (30.4%) and intensive care unit (28.6%). The most frequently isolated mold species was Aspergillus niger (47.2%), followed by Aspergillus flavus (30.6%), Mucor spp. (21.3%), and Aspergillus fumigatus (0.9%). Among the yeasts, the most frequently identified was Candida albicans (50%), followed by Candida krusei (25%) and Candida parapsilosis (25%). Fungi identification was more frequent before sterilization (64.3%) than after opening sterile sets (35.7%).
Conclusion: This study revealed the presence of fungi on small surgical instruments before sterilization and after opening random sterile sets. Implementing advanced environmental control, improving sterilization steps, and monitoring may help reduce nosocomial fungal infections from surgical instruments.
Keywords
Aspergillus spp. Candida spp. Fungal carriage Surgical instruments
The hospital environment being an ecological niche for microorganisms can be a public health concern. Contamin- ation varies among healthcare centers and is related to services, patients, healthcare, and pathogen virulence1. Nosocomial infections can increase hospital morbidity and mortality rates and become burdensome, especially in high-risk departments, for patients who are extremely vulnerable to contamination2,3.
Hospitals offer an ecosystem conducive to the spread of microorganisms, particularly microscopic fungi, which pose a threat to immunocompromised patients4,5 and can be a challenge for clinicians and microbiologists. Moreover, reusable surgical instruments are a potential source of healthcare-associated infections6. Decreased risk of surgical instrument-related infections depends on washing and sterilization pro- cedures. Indeed, staff behavior, poor hygiene, and inadequate training in good hospital hygiene practices can lead to nosocomial fungal infections7. Small surgical instruments used in medical care must be free of microorganisms to avoid nosocomial infections8. The fungi frequently implicated in nosocomial infections are Aspergillus and Candida species5,9. Disinfection and sterilization techniques can reduce pathogen contamination10. Furthermore, an increased incidence of nosocomial fungal infections has been reported worldwide, most likely due to the widespread use of aggressive treatments. The emergence of abundant fungi and variable resistance to antifungal drugs can lead to failure in patient treatment7,11. The effectiveness of control practices depends on the quality of chemical products and compliance with manufacturer's instructions12. In Côte d'Ivoire, data and knowledge of fungal contamination of small surgical instruments are scarce. This study aimed to assess fungal carriage in small surgical instru- ments at a tertiary university hospital in Angré, Côte d'Ivoire.
1. Study design and setting
This descriptive cross-sectional study was carried out for 6 months from February 2024 to July 2024 at a tertiary university hospital, which caters to patients from all muni- cipalities of Abidjan and nearby cities in Angré in Côte d'Ivoire and boasts high-quality technical facilities, modern healthcare infrastructure, and a wide range of services. Samples were collected from small surgical equipment from six services, in- cluding gynecology and obstetrics; intensive care unit (ICU); ear, nose, and throat (ENT) service; dental service; operating block; and surgical emergency, and brought to the parasitology-mycology laboratory for further analyses.
2. Sample collection
Each small surgical instrument, such as scissors, forceps, retractors, and delivery and episiotomy sets, from the sterili- zation unit of each service was sampled using two sterile swabs. Swabbing was performed during disinfection, particularly during the washing stage. After sterilization, one sterile set from each service was randomly selected; swabbing was performed on selected instruments in a sterile hood to avoid contamination. The method used was pressure steam sterilization, which is commonly used for reusable medical devices and is suitable for medical devices, such as textiles, stainless steel devices, plastics, and glass. Using an enclosed autoclave, steam is produced by boiling water under pressure to raise the temperature to 134℃ for 18 min. Steam hydro- lyzes bacteria and fungi, while heat and humidity destroy pathogens by coagulation.
3. Mycological analysis
1) Direct examination and isolation
Two sterile swabs were used to obtain samples for direct examination and culture, respectively. After swabbing the sample on a slide, a drop of physiological water was placed before placing a coverslip for direct examination of fungal elements using light microscopy. Culture was performed on Sabouraud dextrose agar (SDA) medium with chloramphenicol and incubated at 37℃ for 2~5 days, which was sufficient to determine the level of fungal contamination.
2) Fungal identification
Fungal species were identified macroscopically, micro- scopically, and by an automatic machine in difficult cases. Yeast colonies were whitish, smooth, hairless, moist, shiny, or matte. Mold colonies were downy, wooly, or cottony and exhibited varying colors, depending on the species. Yeasts were identified using chromogenic medium (ChromagarTM Candida) and, if with diagnostic difficulty, VITEK 2 Compact® (Biomerieux), according to the manufacturers' protocols. The molds isolated from positive cultures were identified based on microscopic characteristics on lactophenol blue staining between the slide and coverslip. The Aspergillus genus was identified using the conidiophore characteristics of the species with Aspergillus conidial heads (uniseriate or biseriate conidial heads), phialides, and conidia.
4. Data analysis
All statistical analyses were conducted using IBM SPSS Statistics version 21 (Armonk, NY, USA). A patient was con- sidered positive when at least one colony was detected on SDA medium containing chloramphenicol. The variables were analyzed using chi-square and Fisher's exact tests, with an alpha risk of 5%. A p-value of <0.05 was considered statistically significant.
1. Characteristics of surgical instruments
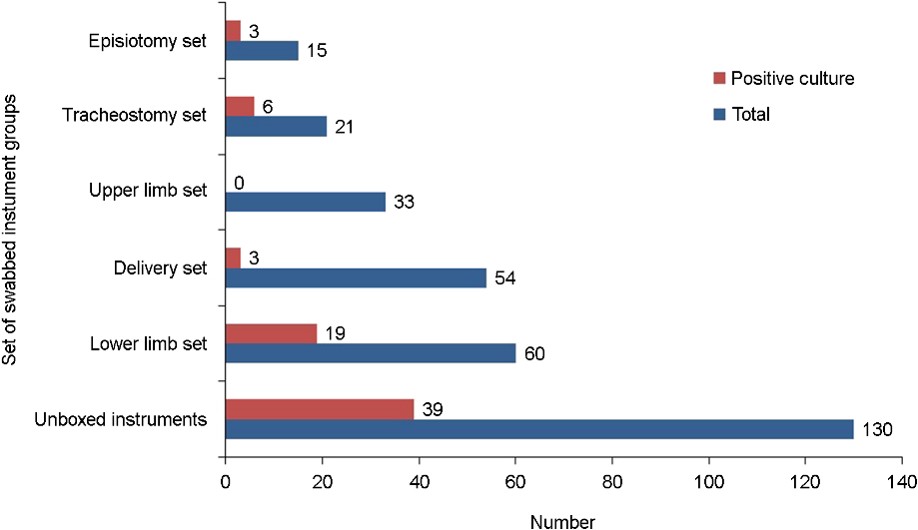
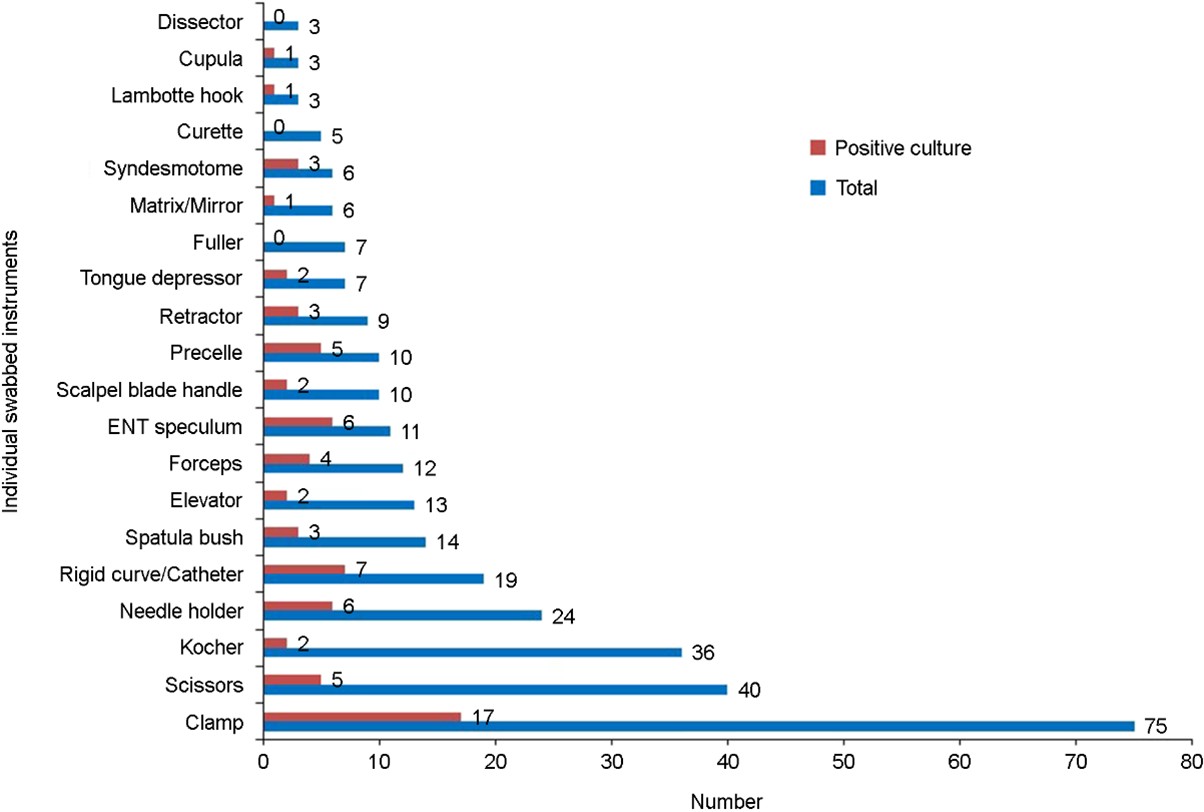
A total of 313 samples were collected from small surgical instruments in the sterilization units of the services. Samples were unevenly distributed among the services, with the operating block having the largest proportion (n = 93, 29.7%), followed by the dental service (n = 79, 25.2%) and gynecology and obstetrics service (n = 69, 22.0%). The surgical emer- gency, ENT, and ICU services had the lowest proportions at 8.9%, 7.3%, and 6.7%, respectively. According to timing, samples were collected before sterilization (n = 217, 69.3%) and after opening random sterile sets (n = 96, 30.7%). Among the small surgical instrument groups, the proportion of collected samples was the highest in the unboxed group (surgical instruments from ENT, dental, and surgical emergency services, n = 130, 41.5%), followed by the lower limb group (n = 60, 19.2%) (Fig. 1). The instruments included in this study were clamps (n = 75, 24.0%); scissors (n = 40, 12.8%); Kocher forceps (n = 36, 11.5%); and needle holders (n = 24, 7.7%) (Fig. 2).
2. Mycological aspects
Of 313 samples, 70 were culture-positive with an overall colonization rate of 22.4%. Among the positive samples, the sampling timing was before sterilization in 64.3% and after opening random sterile sets in 35.7%. Non-boxed samples had the highest number of positive samples (p = 0.0008) (Fig. 1). In total, 70 small surgical instruments showed fungal contamination before sterilization and after opening random sterile sets. The contaminated instruments included clamps (n = 17, 22.7%); catheters (n = 7, 36.8%); needle holders (n = 6, 25.0%); ENT speculums (n = 6, 54.5%); and precelle instruments (n = 5, 50.0%). No fungi were identified on Fuller forceps, curettes, and dissectors (Fig. 2). Furthermore, among the sterile sets opened randomly, the contamination rates among the different services were 37.0% (n = 10) for the operating block, 14.8% (n = 5) for dental service, 14.8% for ICU, 22.2% (n = 3) for ENT, 22.2% (n = 3) for surgical emergency, and 0.4% (n =1) for obstetrics and gynecology.
The most frequently isolated fungi were Aspergillus genus (n = 85, 83.3%), followed by Mucor genus (n = 13, 12.7%) and yeast (n = 4, 4.0%). The most frequently isolated fungal species were Aspergillus niger and Aspergillus flavus. Aspergillus fumigatus was isolated from one instrument (2.9%) from the operating block before sterilization. In the yeast group, Candida parapsilosis (20.0%), Candida albicans (2.9%), and Candida krusei (2.6%) were most frequently identified in instruments from the operating block, dental, and obstetrics and gynecology services, respectively. C. albicans was isolated from one instrument (33.3%) in the sterile set opened randomly from the surgical emergency service (Table 1). As shown in Table 2, clamps were the most frequently contaminated small surgical instruments by A. niger (n = 10, 43.5%); A. flavus (n = 7, 30.4%); and Mucor spp. (n = 3, 13.0%); catheters and needle handles were second and third, respectively.
|
Services |
Fungal
species |
Total n |
Before
sterilization n
(%) |
After
sterilization n
(%) |
p-value |
|
Operating block |
Aspergillus flavus |
11 |
6
(54.5) |
5
(45.5) |
0.670 |
|
Aspergillus fumigatus |
1 |
1
(100) |
0 |
- |
|
|
Aspergillus niger |
13 |
9
(69.2) |
4
(30.8) |
0.0499 |
|
|
Mucor spp. |
9 |
8
(88.9) |
1
(11.1) |
< 0.001 |
|
|
Candida albicans |
1 |
1
(100) |
0 |
- |
|
|
Total |
35 |
25 (71.4) |
10 (28.6) |
|
|
|
Dental service |
Aspergillus flavus |
10 |
8
(80.0) |
2
(20.0) |
0.007 |
|
Aspergillus niger |
21 |
19
(90.5) |
2
(9.5) |
< 0.001 |
|
|
Mucor spp. |
11 |
10
(90.9) |
1
(9.1) |
< 0.001 |
|
|
Candida krusei |
1 |
1
(100) |
0 |
- |
|
|
Total |
43 |
38 (88.4) |
5 (11.6) |
|
|
|
Obstetrical gynecology |
Aspergillus flavus |
1 |
1
(100) |
0 |
- |
|
Aspergillus niger |
4 |
3
(75.0) |
1
(25.0) |
0.160 |
|
|
Candida parapsilosis |
1 |
1
(100) |
0 |
|
|
|
Total |
6 |
5 (83.3) |
1 (16.7) |
|
|
|
Ear, nose, and throat |
Aspergillus flavus |
8 |
6
(85.7) |
2
(14.3) |
0.0455 |
|
Aspergillus niger |
3 |
2
(66.7) |
1
(33.3) |
0.160 |
|
|
Mucor spp. |
3 |
2
(66.7) |
1
(33.3) |
0.160 |
|
|
Total |
14 |
10 (71.4) |
4 (28.6) |
|
|
|
Intensive care unit |
Aspergillus flavus |
2 |
1
(50.0) |
1
(50.0) |
1 |
|
Aspergillus niger |
5 |
2
(40.0) |
3
(60.0) |
0.152 |
|
|
Total |
7 |
3 (42.9) |
4 (57.1) |
|
|
|
Surgical emergency |
Aspergillus flavus |
2 |
2
(100.0) |
0 |
- |
|
Aspergillus niger |
5 |
3
(60.0) |
2
(40.0) |
0.530 |
|
|
Candida albicans |
1 |
0 |
1
(100) |
- |
|
|
Total |
8 |
5 (62.5) |
3 (37.5) |
|
|
Individual |
A. niger |
A. flavus |
A. fumigatus |
Mucor |
C. albicans |
C. krusei |
C. parapsilosis |
Total |
|
Clamp |
10 |
7 |
1 |
3 |
1 |
0 |
1 |
23 |
|
Scissors |
2 |
2 |
0 |
0 |
1 |
0 |
0 |
5 |
|
Kocher |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
|
Needle holder |
5 |
3 |
0 |
1 |
0 |
1 |
0 |
10 |
|
Rigid curve/catheter |
6 |
3 |
0 |
4 |
0 |
0 |
0 |
13 |
|
Spatula brush |
3 |
1 |
0 |
0 |
0 |
0 |
0 |
4 |
|
Elevator |
1 |
2 |
0 |
0 |
0 |
0 |
0 |
3 |
|
Forceps |
4 |
2 |
0 |
2 |
0 |
0 |
0 |
8 |
|
ENT speculum |
3 |
5 |
0 |
1 |
0 |
0 |
0 |
9 |
|
Scalpel blade handle |
2 |
0 |
0 |
2 |
0 |
0 |
0 |
4 |
|
Precelle |
5 |
1 |
0 |
2 |
0 |
0 |
0 |
8 |
|
Retractor |
1 |
2 |
0 |
1 |
0 |
0 |
0 |
4 |
|
Tongue depressor |
0 |
2 |
0 |
2 |
0 |
0 |
0 |
4 |
|
Fuller |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Matrix/mirror |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
2 |
|
Syndesmotome |
3 |
1 |
0 |
2 |
0 |
0 |
0 |
6 |
|
Curette |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Lambotte hook |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
2 |
|
Cupula |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
2 |
|
Dissector |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Total |
50 |
32 |
1 |
22 |
2 |
1 |
1 |
|
This cross-sectional study revealed an unequal distribution of fungal carriage in small surgical instruments among the clinical services of a tertiary hospital in Côte d'Ivoire. Microbial contamination of surgical instruments can have serious consequences for patients in healthcare facilities13. Among the various microorganisms that may contaminate surgical instruments, fungi are a real major concern for healthcare establishments and practitioners. These pathogens thrive in warm and humid environments, which are common in operating blocks and healthcare rooms. Hence, they pose a threat of soiling and infection from surgical instruments. Despite the cleaning and sterilization of surgical instruments, fungal contamination remains a problem in healthcare estab- lishments worldwide14,15. This study revealed an overall fungal contamination rate of 22.4%.
Similar findings of fungal contamination of surgical instru- ments from operating theaters at the time of opening of surgical sets have been reported16. These results underscore the importance of continuous monitoring for fungal contam- ination in clinical environments. Contamination can be due to several factors, including disinfection practices, equipment storage time, and surgical environment. Inadequate cleaning procedures often explain the variations in fungal contam- ination rates, underscoring the need to improve existing protocols17.
This study revealed fungal colonization before sterilization and after random opening of sterile sets. Unbalanced sample sizes could lead to misinterpretations of the distribution of fungi among the services; those with smaller sample sizes may appear to have higher contamination rates. This result raises concerns about the effectiveness of sterilization methods. Furthermore, some sterilization methods may not effectively eliminate all types of fungi, particularly resistant spores18.
At our institution, surgical instruments are sterilized using steam with pressure or moist heat sterilization, which is commonly used for reusable medical devices. The efficacy of sterilization depends on effective destruction of living organisms16. Similar to a previous study19, our study used a combination of chemical products, including chlorhexidine gluconate and ethanol, for sterilization in order to prevent dissemination of infectious pathogens. Factors, such as residual humidity, inappropriate handling after sterilization, and lack of predisinfection steps, among the services can enhance fungal resistance12,20,21. This could have significant clinical consequences, such as increased risk of nosocomial infections. Therefore, sterility should be maintained from sterilization to handling and use of instruments in surgical settings.
Our study revealed a predominance of molds in surgical instruments. These pathogens are common in hospital envir- onments because of their resistance and ability to grow in favorable conditions. Previous studies have shown a high presence of molds in ___, highlighting the need for control strategies to reduce contamination13,17,22. Notably, molds can have significant consequences on patient health by increasing the risk of nosocomial infections.
Among the fungal species identified, A. niger was the most frequently isolated among all services in this study. Other studies have reported that mold species were commonly found in hospital environments9,13. Another identified con- taminant was A. fumigatus, which can cause invasive respira- tory tract infections in >80%. It plays an important role in opportunistic nosocomial infections, frequently affecting the lungs because of the small size of spores, which can easily reach pulmonary alveoli23,24. The highest rates of fungal contamination were observed in the operating block and dental services, suggesting that these clinical areas were more prone to fungal growth. Departments with frequent hand-ling and circulation of surgical instruments are particularly vulnerable to contamination, particularly when hygiene and sterilization practices are suboptimal16.
In terms of yeast contamination, C. albicans was frequently identified among the clinical services. Previous studies classified C. albicans as a fungal species that has been mostly isolated from nosocomial candidemia25,26. However, over the last decade, we have witnessed the emergence of nonalbicans species27,28. Indeed, the epidemiology of candidiasis has changed considerably in recent years, with the appearance of nonalbicans species in hospitals26. C. parapsilosis, which was also identified in our study, has become one of the main causes of nosocomial candidemia over the last decade26,29. It is a saprophytic yeast on the skin and is characterized by its affinity for catheters and other small instruments, leading to a wide range of infections with dreadful prognoses. The presence of these yeasts in hospital environments may be associated with their ability to survive on plastic surfaces for extended periods and the use of reusable medical devices16. Candida auris was not identified in this study. This yeast is capable of causing nosocomial infections in immunocompro- mised persons; it is associated with candidemia with high mortality rate and presents a serious global health threat30.
The most frequently contaminated surgical instruments were clamps, catheters, needle holders, and speculums. Similar results for surgical instruments in the operating rooms of hospitals in Iran were recently reported16. The main reason for contamination of these instruments could be prolonged contact with the operating room environment after opening the sterile packs or the frequency of use. These instruments are often unused and may be exposed to environmental contamination by airborne fungal spores, which can deposit on surfaces and instruments over time31,32. This can increase the rate of various diseases, including allergic reactions; infec- tious diseases, such as aspergillosis; toxicosis; and respiratory ailments, such as asthma, hypersensitivity, and pneumonitis. The impact of air quality on fungal contamination due to suspended particles is a major source of air pollution33,34. Reducing the infectious risk associated with reusable medical devices requires the implementation of effective and rigorous treatment and procedures by trained personnel.
Members of the staff who are primarily involved in dis- infection and sterilization need appropriate qualifications, including continuing education at regular intervals and periodic competency assessment35. Sterilization is one of the effective processes for eliminating pathogenic microorganisms from reusable medical devices. This process is described as special, because the sterility of a product cannot be verified without compromising it. The fact that the survival of a microorganism after sterilization is highly unlikely calls into question the procedure for the various stages of sterilization. Compliance with instructions in the predisinfection zone and distribution of instruments in the machine are important steps to guar- antee better sterilization of surgical instruments36.
This study had some limitations. The methods of identifi-cation were microscopy for molds and chromogenic medium and VITEK 2 for yeasts. Therefore, emerging pathogens, such as nonalbicans Candida species (e.g., Candida auris) and related species (e.g., Candida duobushaemulonii and Candida haemulonii), might have been missed. To improve the precision of fungal identification, MALDI-TOF MS and PCR-based methods are necessary. However, these techniques were not used in this study.
The findings of this study highlighted an important aspect of handling surgical instruments beyond the initial sterilization process. A. niger and C. albicans were the most commonly identified species. Prevention of fungal contamination of surgical instruments requires the implementation of control strategies and microbiological surveillance. Moreover, im-proving sterilization techniques and regular monitoring in healthcare facilities could enhance patients' security against nosocomial fungal infections transmitted by surgical instru- ments.
References
1. Cavallo J, Antoniotti G, Baffo N, Condrais G, Hajjar J, Horn C, et al. Microbiological surveillance of the environ- ment in healthcare establishments: air, water and sur- faces. Department of hospitalization and healthcare organization, CTIN, 2002;70
2. Chouchene I, Bouafia N, Ben Cheikh A, Toumi B, Mahjoub M, Bannour W, et al. Incidence of device-associated infections in a Tunisian intensive care unit. Sante Publique 2015;27:69-78
Google Scholar
3. Merzougui L, Barhoumi T, Guizani T, Barhoumi H, Hannachi H, Turki E, et al. Nosocomial infections in the intensive care unit: annual incidence and clinical aspects, Tunisia, 2014. Pan Afr Med J 2018;30:143
Google Scholar
4. Diongue K, Badiane AS, Seck MC, Ndiaye M, Diallo MA, Dialo S, et al. Qualitative fungal composition of services at risk of nosocomial infections at Aristide Le Dantec Hospital (Dakar). J Mycol Med 2015;25:e39-43
Google Scholar
5. Monemo P, Adoubryn K. Environmental microbiological study of the intensive care unit: Results of a preliminary survey. University of Cocody, Medical Sciences Unit Abidjan, 2016:56
6. Khomsi Z, El Marnissi S, El Harti J, Allou KR. Risk manage- ment mapping of medical device sterilization excluding washing. The case of the central sterilization unit at Ibn Sina Hospital in Rabat. Hospital Pharmacist and Clinician 2019;54:241-249
7. French Society of Hospital Hygiene. Monitoring and pre- venting healthcare-associated infections. University of Claude-Bernard, France 2010:165
8. WHO. Monitoring and preventing healthcare-associated infections: what measures to adopt in anesthesia. https://www.sf2h.net/publications/surveiller-et-prevenir-les-infections-associees-aux-soins.html
9. Prakash PY. Fungal surgical site infections. Int Wound J 2014;13:428
Google Scholar
10. Dettenkofer M. Healthcare environment decontamination. Healthcare Infection 2013;18:47-48
Google Scholar
11. Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018;360:739-742
Google Scholar
12. Darbord J, Dumartin C, Dumartin M. Disinfection and sterilization in health care facilities: A practical guide. 5th ed. Nursing knowledge and practice. Masson: Paris 2003:65
13. Macedo CE, Ferreira AM, Barcelos LD, Alvim AL, Carneiro LM, Martins SR, et al. Contamination of equipment and surfaces in the operating room anesthesia workspace: A cross-sectional study. Sao Paulo Med J 2024;142: e2023177
Google Scholar
14. Bonadonna L, Briancesco R, Coccia, AM. Analysis of microorganisms in hospital environments and potential risks. In indoor air quality in healthcare facilities. Cham: Springer, 2017:53-62
Google Scholar
15. Sznajder W, Jankowska-Polańska B, Tański W. A narrative review of fungal periprosthetic joint infections of the hip and knee: Risk factors, microbiological profiles, and treatment challenges. J Clin Med 2025;14:206
Google Scholar
16. Danesh HA, Arasteh H, Keikha N, Haghighi JD, Aslani P. Investigation the frequency of fungal contamination in surgical instruments. Ann Mil Health Sci Res 2024;22: e150785
Google Scholar
17. Obasi C, Agwu A, Akinpelu W, Hammons R, Clark C, Etienne-Cummings R, et al. Contamination of equipment in emergency settings: An exploratory study with a targeted automated intervention. Ann Surg Innov Res 2009;3:8
Google Scholar
18. Garvey M, Kremer TA, Rowan NJ. Efficacy of cleaning, disinfection, and sterilization modalities for addressing infectious drug-resistant fungi: A review. J Appl Microbiol 2025;136:lxaf005
Google Scholar
19. Tweij-Thu-Alfeqar Razzaq AJ, Shnan D, Ali AB. Sterili- zation of surgical tools: Removing bacterial endospores with a combination of povidone-iodine, chlorhexidine gluconate, ethanol, and methanol. J Pure Appl Microbiol 2019;13:2499-2506
Google Scholar
20. Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: Chemical composition and role in drug resistance. J Med Microbiol 2006;55: 999-1008
Google Scholar
21. Cheikh MHB, Teka M, Fhal M, Zribi K, Majdoub A. Mise en place d'un système de management de la qualité dans une unité de stérilisation centralisée: expérience de l'Hôpital Tahar Sfar de Mahdia en Tunisie. Pan Afr Med J 2021;39:287
22. Perdelli F, Cristina ML, Sartini M, Spagnolo AM, Dallera M, Ottria G, et al. Fungal contamination in hospital environments. Infect Control Hosp Epidemiol 2006;27: 44-47
Google Scholar
23. Lugauskas A, Sveistyte L, Ulevicius V. Concentration and species diversity of airborne fungi near busy streets in lithuanian urban areas. Ann Agric Environ Med 2003; 10:233-239
Google Scholar
24. Earle K, Valero C, Conn DP, Vere G, Cook PC, Bromley MJ, et al. Pathogenicity and virulence of Aspergillus fumigatus. Virulence 2023;14:2172264
Google Scholar
25. Matotou HR, Sangare I, Bisseye C, Akotet MK, Bamba S. Biodiversité de la flore fongique isolée au service de réanimation du Centre Hospitalo-Universitaire Souro Sanou de Bobo-Dioulasso, Burkina Faso. Pan Afr Med J 2021;38:299
Google Scholar
26. Ortiz B, Aguilar K, Galindo C, Molina L, Fontecha G. Candida species isolated from clinical samples in a tertiary hospital in Honduras: Where is Candida auris? Curr Med Mycol 2022;8:1-8
Google Scholar
27. Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One 2011;6: e24198
Google Scholar
28. Husni R, Bou-Zerdan M, Samaha N, Helou M, Mahfouz Y, Saniour R, et al. Characterization and susceptibility of non-albicans Candida isolated from various clinical speci- mens in Lebanese Hospitals. Front Public Health 2023; 11:1115055
Google Scholar
29. Pfaller MA, Diekema D. Epidemiology of invasive can- didiasis: A persistent public health problem. Clin Microbiol Rev 2007;20:133-163
Google Scholar
30. Dahiya S, Chhillar AK, Sharma N, Choudhary P, Punia A, Balhara M, et al. Candida auris and nosocomial infection. Curr Drug Targets 2020;21:365-373
Google Scholar
31. Górny RL, Reponen T, Willeke K, Schmechel D, Robine E, Boissier M, et al. Fungal fragments as indoor air biocontaminants. Appl Environ Microbiol 2002;68:3522-3531
Google Scholar
32. Jürgensen CW, Madsen AM. Influence of everyday activities and presence of people in common indoor environments on exposure to airborne fungi. AIMS Environmental Science 2016;3:77-95
Google Scholar
33. Whyte W, Ward S, Whyte WM, Eaton T. Decay of airborne contamination and ventilation effectiveness of cleanrooms. Int J Vent 2014;13:211-220
Google Scholar
34. Ghajari A, Lotfali E, Azari M, Fateh R, Kalantary S. Fungal airborne contamination as a serious threat for respiratory infection in the hematology ward. Tanaffos 2015;14:257-261
Google Scholar
35. WHO. Decontamination and reprocessing of medical devices for healthcare facilities: Aide-memoire. World Health Organization: Geneva, Switzerland, 2020
36. Simões Sde A, Leite Júnior DP, Hahn RC. Fungal microbiota in air-conditioning installed in both adult and neonatal intensive treatment units and their impact in two university hospitals of the central western region, Mato Grosso, Brazil. Mycopathologia 2011;172:109-116
Google Scholar
Congratulatory MessageClick here!