pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Sumati Hogade,Sneha Chavan,Vaibhavi Verekar,Ahuti Pandya,Anandkumar R Annigeri,Tejashwar Hogade
10.17966/JMI.2024.29.4.181 Epub 2025 January 03
Abstract
Background: Tinea incognito refers to the unusual clinical manifestation of dermatophytosis brought on by prior topical or systemic medication use. Given the widespread and easy diagnosis of dermatophytic infections, steroid usage has created a diagnostic conundrum, thus turning a straightforward infection that may be treated into a persistent, long-term skin disease.
Objective: To examine the clinical characteristics of tinea incognito and correlate the clinical manifestations with the microbiological findings.
Methods: An observational study was conducted with 100 cases in a department of microbiology, dermatology, venereology, and leprology, in India. The baseline data of the patients and clinical features of tinea incognito were recorded. To identify the causative agents, skin scrapes were collected and tested as per standard protocol.
Results: In this study, 30% of the patients were between 31 and 40 years old. The male-to-female ratio was 2:1, 66% reported a history of application of the triple combination of steroids, and 34% used single steroids. Tinea corporis was seen in 30% of the patients, and the trunk/body (52%) was the most frequent site, followed by other sites (34%). Potassium hydroxide positivity was observed in 75% of the cases, cultural positivity in 77%, and both tests were positive in 75%. The most common organisms found were T. mentagrophytes (72.7 (56/77)%), T. rubrum (19.5 (15/77)%), and T. tonsurens 7.8 (6/77)%.
Conclusion: Tinea incognito is a fungal skin infection that can present with various nonspecific clinical features. This steroid-modified dermatophytosis is largely under-reported because the underlying fungal infection is masked by the irrational use of topical steroids.
Keywords
Dermatophytosis Steroid Tinea incognito Trichophyton spp.
Tinea incognito is a dermatophyte infection altered by corticosteroid therapy1. Dead keratin is metabolized by dermatophytes, which causes dermatitis. This reaction reduces the amount of keratin that the fungus receives, thus suppressing the infection. Steroids and other immunosuppressants inhibit this protective eczematous response, which promotes fungal growth2. Patients with these infections can be of any age or sex. Although it can affect any area, it most commonly occurs in the face and arms3. Risk factors of infection include occupational hazards, excessive sweating, poor hygiene, immunocompromised status, lack of basic education among people of low socioeconomic status, overcrowding, humid conditions, and excessive exposure to outdoor activities such as agriculture and manual labor4-6. The clinical presentation of tinea incognito varies widely and can be mistaken for skin conditions, such as psoriasis, erythema migrans, lichen planus, seborrheic dermatitis, eczema, and purpura7-9. Lesions are typically unusual; thus, its diagnosis is sometimes overlooked or delayed. The diagnosis of tinea incognito can be easily done by using potassium hydroxide (KOH) preparation to visualize branching hyphae and spores, which are characteristic of dermatophytes10. Treatment of tinea incognito consists of discontinuing all topical steroids and starting a targeted anti- fungal regimen. The daily incidence of such cases is increasing because of the increased availability and use of over-the-counter medications and topical steroids. To link the clinical manifestations with the microbiological findings, this study aimed to investigate the clinical and mycological aspects of tinea incognito.
This cross-sectional study was conducted for 1 year after obtaining institutional ethical committee clearance. Written informed consent mentioning confidentiality was obtained from the patient.
The study included a total, 100 patients of both sexes with clinical characteristics suggestive of tinea incognito (clinically, these lesions have less raised margin and are not scaly as classic dermatophytosis; they tend to be extensive, pruritic, erythematous, and pustular) and a history of steroid overuse. Skin scraping from the edges of the skin lesions was taken and then subjected to 10% KOH preparation. Samples were also inoculated onto Sabouraud dextrose agar (SDA) for culturing. By analyzing colony morphology and pigment production, species growing from SDA plates were identified by micro- scopic examination with lactophenol cotton blue (LPCB). For final identification, conventional procedures were performed to conduct tests such as the hair perforation test, urease test, and slide culture, as needed.
1. Statistical analysis
A Microsoft Excel spreadsheet was used to record the collected data. Continuous data (SD) were expressed as means and SDs. Categorical data were expressed as rates, ratios, and percentages and were compared using the chi-square test, test of proportion, and Fisher's exact test. A probability value of ≤0.050 indicates significance.
This cross-sectional study was conducted from January 2020 to December 2020. A total of 100 patients with clinical signs and symptoms suggestive of tinea incognito were included. Clinically, these lesions have a less raised margin and are not as scaly as classic dermatophytosis. They tend to be extensive, pruritic, erythematous, and pustular (Fig. 1).
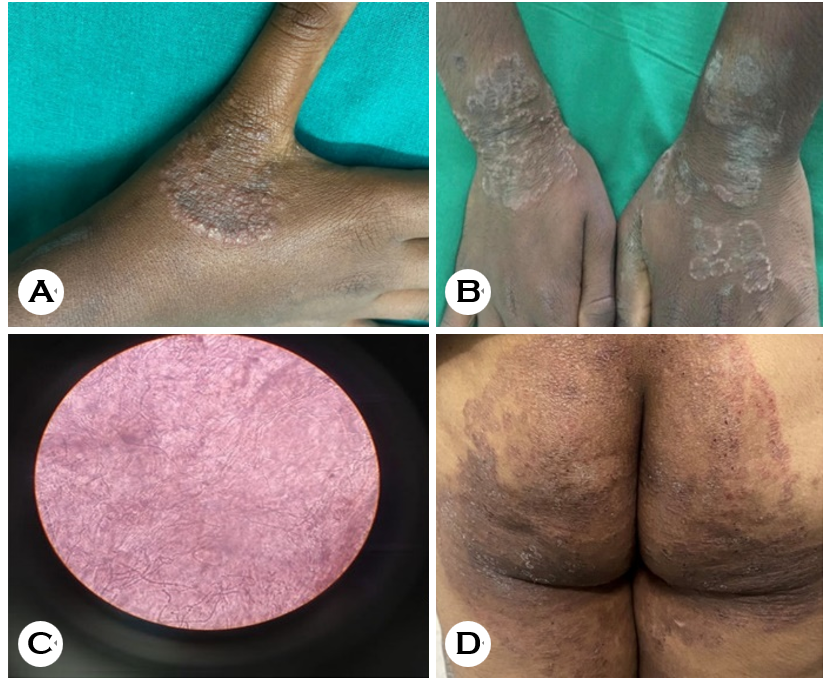
In this study, 61% of the patients were male, and 39% were female, with a male-to-female ratio of 2:1. Patients aged 31~40 years were predominant, comprising 30% of the patients (male = 20%; female = 10%), followed by those aged 21~30 (28%), 41~50 (26%), 51~60 (12%), <20 (3%), and 61~70 (1%) respectively. Moreover, 61% of the patients belong to socioeconomic class III (middle class), followed by those in the upper middle class (29%) according to the BJ Prasad revised classification (Table 1).
|
Age |
No. of |
Sex of the
patient |
|
Socioeconomic
status of the patient* |
|||||
|
Male |
Female
|
I |
II |
III |
IV |
V |
|||
|
<20 |
3 |
2 |
1 |
|
0 |
1 |
0 |
2 |
0 |
|
21~30 |
28 |
18 |
10 |
|
1 |
10 |
16 |
1 |
0 |
|
31~40 |
30 |
20 |
10 |
|
0 |
10 |
16 |
3 |
1 |
|
41~50 |
26 |
13 |
13 |
|
0 |
3 |
21 |
2 |
0 |
|
51~60 |
12 |
7 |
5 |
|
0 |
5 |
7 |
0 |
0 |
|
61~70 |
1 |
1 |
0 |
|
0 |
0 |
1 |
0 |
0 |
|
Total |
100 |
61 |
39 |
|
1 |
29 |
61 |
8 |
1 |
|
*On
the basis of BG Prasad's revised socioeconomic status classification (2021) |
|||||||||
In addition, 66% of the patients reported a history of appli- cation of triple combination of drugs (ofloxacin, ornidazole, terbinafine hydrochloride, and clobetasol propionate cream), followed by 34% who reported single steroid use (beclo- methasone dipropionate 0.025%, followed by clobetasol propionate 0.05%, and fluocinolone acteonid 0.01%). The duration of steroid application was 7~12 months in 67% of the patients, with a minimum of 5 months and a maximum of 18 months (Table 2).
|
Corticosteroid application |
|
Distribution (n = 100) |
|
|
Number |
Percentage |
||
|
Duration |
<6
months |
14 |
14 |
|
|
7~12
months |
67 |
67 |
|
|
13~18
months |
19 |
19 |
|
Steroids used |
|
|
|
|
|
Triple
combination* |
66 |
66 |
|
|
Single steroid |
34 |
34 |
|
|
1. Beclomethasone dipropionate 0.025% |
20 |
58.8 |
|
|
2. Clobetasol propionate 0.05% |
9 |
26.5 |
|
|
3. Fluocinolone acetonide 0.01% |
5 |
14.7 |
|
*Triple combination includes ofloxacin, ornidazole, terbinafine
hydrochloride, and clobestosol propionate cream |
|||
In this study, lesions were found on the trunk/body (52%), face (14%), and other sites (34%). The most common clinical presentation was an ill-defined erythematous plaque with less scaling (56%), followed by psoriatic lesions (19%), various other presentations such as eczemas (18%), and other atypical presentations (rosacea, discoid lupus erythematosus, and impetigo) (7%). The most common fungal species isol- ated was T. mentagrophytes (56%), followed by T. rubrum (15%), T. tonsurans (6%), and no growth was found in 23% (Table 3).
|
Clinical presentation |
Site of the Lesion |
|
Mycological growth |
|||||
|
Face |
Trunk/ |
Other |
T. mentagrophytes |
T. rubrum |
T. tonsurans |
No |
||
|
Eczematous
lesion n=18 |
4 |
9 |
5 |
|
5 |
2 |
0 |
11 |
|
Psoriatic
lesion n=19 |
3 |
11 |
5 |
|
10 |
2 |
4 |
3 |
|
Hyperpigmented n=56 |
6 |
30 |
20 |
|
34 |
11 |
2 |
9 |
|
Other atypical lesions n=7 |
1 |
2 |
4 |
|
7 |
0 |
0 |
0 |
|
Total n=100 |
14 |
52 |
34 |
|
56 |
15 |
6 |
23 |
In this study, fungal elements were observed in 75% (75/100) of the cases in the KOH test, cultures were positive in 77% (77/100), and both KOH tests and culture were positive in 75% (75/100). By Cohen's kappa, 0.945 (p-value) showed a substantial agreement between the results of the KOH test and culture, and the agreement between these two was 98%. The diagnostic parameters of KOH over culture showed a sensitivity of 97.4%, a specificity of 100%, a positive-predictive value of 100%, and a negative-predictive value of 92%.
In this study, patients were taking oral and topical anti- fungals for 2 months, from which the patients exhibited significant improvements with no recurrence.
This study analyzed patients aged >18 years with skin lesions and a history of steroid use. The age group most frequently affected was those aged 31~40 years 30%. In this study, 28% of the patients were 21~30 years old and <1% were 61~70 years old. This result aligns with the results of Bhatia et al.11, reporting 21~50 years (64.9%) as the mean age. In another study by Maulingkar et al.12, the mean age group was the third decade (28%). The present study had a male predominance. The male-to-female ratio was 2:1 in the present study and that in the study by Pathania et al.13 was 1.7:1 (63% vs 37%). In another study by Rudramurthy et al.14, the male-to-female ratio was 2.8:1 (73.8% vs 26.1%) and that in the study by Bhatia et al.11 was 5.7:1. Men have historically been afflicted more often than women, which was most likely due to their propensity for outdoor employment that exposes them to hot, muggy, and sweaty environments that increases the development of dermatophytosis.
In this study, 61% of the patients belonged to the middle socioeconomic strata; this result matched the findings of Hanumanthappa et al.15, who reported that the majority of dermatophyte infections occurred in those in the lowest socioeconomic strata (65.4%). Poluril et al.16 revealed a high incidence in individuals in the lowest socioeconomic group (67.74%). Noronha et al.17 showed that most patients were from poor social class (61.3%). Thus, belonging to lower socioeconomic groups, unhygienic living conditions, living in crowded quarters, and inadequate nutrition are risk factors for infection and, if not identified, will lead to chronicity and recurrence. In the present study, the average duration of the application of steroids was 7~12 months i.e., 67% (67/100), in comparison with that reported by Ansar et al.18, which was approximately 1 year. Dutta et al.19 reported that the duration of steroid application varied from 6 weeks to 12 months; thus, in the present study, patients continue to use steroids for a longer duration because of the abrupt reduction in pruritis and clearing of lesions. In the present study, 66% of the patients reported using steroid combinations that were sold over the counter (OTC), and 53.2% utilized OTC topical corticosteroid combination in a related study conducted by Pathania et al.13 Mahajan et al.20 and Singh et al.21 revealed that 187 (70.6%) and 63 (42%) patients, respectively, took a combination of topical steroids. The increasing availability of OTC topical steroid creams and their irrational usage were found to be strongly correlated. The present study showed the incorrect and widespread use of preparations containing steroids, as well as their easy availability, low cost, and early alleviation from inflammatory symptoms (such itching), are major contributors to the current revival of dermatophytosis. These drugs inhibit the immunity mediated by the host cell, which makes the disease resistant to therapy22. In this study of tinea incognito, ill-defined erythematous plaque with less scaling was the most common presentation, followed by eczematous lesions. Vineetha et al. reported similar atypical presentations along with eczematous changes23. In a study by Romano et al.24, eczema was the most common clinical manifestation. Dogra et al.25 reported various atypical presen- tations of tinea incognito such as eczema and psoriasis. The trunk (52%) was the most common site of the clinical lesion in our patients. In the study by Thakur et al.26, the clinical lesions were more commonly seen on the trunk (61.20%). Multiple sites and single genital involvement were suggestive of topical steroid usage, as reported by Verma et al.22 KOH test positivity was observed in 75% (75/100) of our cases and culture positivity in 77% (77/100), and both KOH and culture positive were observed in 75% (75/100). This result aligns with the results of Baneriee et al.27, who reported 90% culture positivity and 80.95% KOH positivity. In the study by Noronha et al.28, direct microscopy was positivee in 60%, and 52.4% of patients in the study by Mahajan et al.20 showed cultural positivity and 79.6% indicated KOH positivity.
In the present study, T. mentagrophyte was the most common isolated organism at 56% (56/100), followed by T. rubrum at 15%. Tigga et al.29 showed that T. mentagrophyte accounted for 97.2% of the isolated dermatophytes. Accord- ing to Nenoff et al.30, T. mentagrophyte (48.3%) was the most often occurring organism in culture. Bhatia et al.11 indicated that T. mentagrophyte (63.5%) was the most often emerging organism. T. mentagrophytes grows quickly in 5~7 days, which may account for the inflammatory lesions, extensive involvement, and fomite transmission22.
1. Limitation
The study was an outpatient, hospital-based study with a small sample size and not a complete representation of the population. Thus, the results cannot be generalized to the entire population. We have not performed antifungal sus- ceptibility testing to determine the most sensitive drug against Trichophyton spp., and we did not perform histopathological examination to rule out noninfective manifestations.
The overuse of steroids is the primary cause of the nation's growing epidemic of superficial fungal infections. Physicians, paramedics, and the general public must be made more aware of the negative effects of steroids on fungal infections. Thus, the manufacture and distribution of illogical topical formulations that combine steroids and antifungals must be regulated. The public and medical professionals must be informed about the risks associated with topical steroids, including their serious adverse effects, and the need for prudent and thoughtful use to avoid tinea incognito.
References
1. Solomon BA, Glass AT, Rabbin PE. Tinea incognito and
Google Scholar
2. Ive FA, Marks R. Tinea incognito. Br Med J 1968;3:149-152
Google Scholar
3. Arenas R, Moreno-Coutiño G, Vera L, Welsh O. Tinea in- cognito. Clin Dermatol 2010;28:137-139
Google Scholar
4. Noronha TM, Tophakhane RS, Nadiger S. Clinico-microbiological study of dermatophytosis in a tertiary-care hospital in North Karnataka. Indian Dermatol Online J 2016;7:264-271
Google Scholar
5. Shenoy M. Superficial fungal infections. In: Sacchidanand S, editor. IADVL Textbook of Dermatology, 5th ed. Mumbai: Bhalani Publishing House. 2022:537-538
6. Moriarty B, Hay R, Morris-Jones R. The diagnosis and management of tinea. BMJ 2012;345:e4380
Google Scholar
7. Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses 2006;49:383-387
Google Scholar
8. Agostini G, Knöpfel B, Difonzo EM. Universal dermato- phytosis (tinea incognito) caused by Trichophyton rubrum. Hautarzt 1995;46:190-193
Google Scholar
9. Guenova E, Hoetzenecker W, Schaller M, Rocken M, Fierlbeck G. Tinea incognito hidden under apparently treatment-resistant pemphigus foliaceus. Acta Derm Venereol 2008;88:276-277
Google Scholar
10. Maraki S, Nioti E, Mantadakis E, Tselentis Y. A 7-year survey of dermatophytoses in Crete, Greece. Mycoses 2007;50:481-484
Google Scholar
11. Bhatia VK, Sharma PC. Epidemiological studies on der- matophytosis in human patients in Himachal Pradesh, India. Springer Plus 2014;3:1-7
Google Scholar
12. Maulingkar SV, Pinto MJ, Rodrigues S. A clinico-mycological study of dermatophytoses in Goa, India. Mycopathologia 2014;178:297-301
Google Scholar
13. Pathania S, Rudramurthy SM, Narang T, Saikia UN, Dogra S. A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J Dermatol Venereol Leprol 2018; 84:678-684
Google Scholar
14. Rudramurthy SM, Shaw D. Overview and update on the laboratory diagnosis of dermatophytosis. Clin Dermatol Rev 2017;1:S3-11
Google Scholar
15. Hanumanthappa H, Sarojini K, Shilpashree P, Muddapur SB. Clinicomycological study of 150 cases of dermato- phytosis in a tertiary care hospital in South India. Indian J Dermatol 2012;57:322-323
Google Scholar
16. Poluri LV, Indugula JP, Kondapaneni SL. Clinicomycological study of dermatophytosis in South India. J Lab Physicians 2015;7:84-89
Google Scholar
17. Noronha TM, Tophakhane RS, Nadiger S. Clinico-microbiological study of dermatophytosis in a tertiary-care hospital in North Karnataka. Indian Dermatol Online J 2016;7:264-271
Google Scholar
18. Ansar A, Fershchian M, Naseri H, Ghiasian SA. Clinico-epidemiological and mycological aspects of tinea incognito in Iran: a 16-years study. Med Mycol J 2011;52:25-32
Google Scholar
19. Dutta B, Rasul ES, Boro B. Clinico-epidemiological study of tinea incognito with microbiological correlation. Indian J Dermatol Venereol Leprol 2017;83:326-331
Google Scholar
20. Mahajan S, Tilak R, Kaushal SK, Mishra RN, Pandey SS. Clinicomycological study of dermatophytic infections and their sensitivity to antifungal drugs in a tertiary care center. Indian J Dermatol Venereol Leprol 2017;83:436-440
Google Scholar
21. Singh S, Verma P, Chandra U, Tiwary NK. Risk factors for chronic and chronic-relapsing tinea corporis, tinea cruris and tinea faciei: Results of a case-control study. Indian J Dermatol Venereol Leprol 2019;85:197-200
Google Scholar
22. Verma SB, Panda S, Nenoff P, Singal A, Rudramurthy SM, Uhrlass S, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. Epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol 2021;87:154-175
Google Scholar
23. Vineetha M, Sheeja S, Celine MI, Sadeep MS, Palackal S, Shanimole PE, et al. Profile of dermatophytosis in a tertiary care center. Indian J Dermatol 2018;63:490-495
Google Scholar
24. Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses 2006;49:383-387
Google Scholar
25. Dogra S, Narang T. Emerging atypical and unusual pre- sentations of dermatophytosis in India. Clin Dermatol Rev 2017;1:S12-18
Google Scholar
26. Thakur R, Kalsi AS, Kushwaha P, Singh P. Epidemiology of corticosteroid-modified tinea: Study of 100 cases in a rural tertiary care teaching hospital of Western Uttar Pradesh, India. J Dermatol Cosmetol 2018;2:64-69
Google Scholar
27. Baneriee U, Sharma KA. A Study on Dermatophytosis in Delhi. Indian J Dermatol Venereol Leprol 1984;50:41-44
Google Scholar
28. Noronha TM, Tophakhane RS, Nadiger S. Clinico-microbiological study of dermatophytosis in a tertiary-care hospital in North Karnataka. Indian Dermatol Online J 2016;7:264-271
Google Scholar
29. Tigga RA, Das S, Bhattacharya SN, Saha R, Pandhi D, Datt S, et al. Burden of chronic dermatophytosis in a tertiary care hospital: Interaction of fungal virulence and host immunity. Mycopathologia 2018;183:951-959
Google Scholar
30. Nenoff P, Verma SB, Vasani R, Burmester A, Hipler UC, Wittig F, et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes: A molecular study. Mycoses 2019;62:336-356
Google Scholar
Congratulatory MessageClick here!