pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Ik Jun Moon,Woo Jin Lee,Hyun Chang Ko,Hyojin Kim,Chan Ho Na,Joonsoo Park,Jin Park,Hyun-Min Seo,Min Kyung Shin,Young Bok Lee,Yong Hyun Jang,Hye Jung Jung,Yangwon Lee
10.17966/JMI.2024.29.3.117 Epub 2024 October 11
Abstract
Background: Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause various cutaneous complications, including dermatologic adverse reactions to SARS-CoV-2 vaccines reported by several studies.
Objective: To describe the clinical characteristics of cutaneous complications of SARS-CoV-2 infection and adverse reactions to SARS-CoV-2 vaccines, and to determine the risk factors for cutaneous manifestations.
Methods: A questionnaire-based survey in 12 hospitals in Korea.
Results: After receiving SARS-CoV-2 vaccinations, 20.23% and 5.94% of the respondents reported new-onset cutaneous lesions or aggravation of preexisting cutaneous conditions, respectively. Respondents who developed new cutaneous lesions after COVID-19 were significantly older than those who did not (p = 0.001). Systemic symptoms of SARS-CoV-2 vaccination (fever, chill, cough, sore throat, and myalgia) were associated with higher risk for new-onset cutaneous lesions (p < 0.05). Myalgia was the only systemic symptom of SARS-CoV-2 vaccination that was associated with higher risk for the aggravation of preexisting cutaneous conditions (p = 0.011). Following coronavirus 2019 (COVID-19) diagnosis, 13.3% and 9.7% of the respondents reported new skin lesions and aggravation of preexisting cutaneous conditions, respectively. Respondents with new cutaneous lesions were significantly older than those without new cutaneous lesions (p = 0.046). Systemic COVID-19 symptoms were significantly more common in respondents who developed new cutaneous lesions than in those who did not (p < 0.001). The proportion of respondents with underlying autoimmune diseases was significantly higher in those with cutaneous COVID-19 complications than in those without such complications (p = 0.038).
Conclusion: This study offers insights into the characteristics of cutaneous manifestations of SARS-CoV-2 vaccination and infection in Korea.
Keywords
Coronavirus disease 2019 Severe acute respiratory syndrome coronavirus 2 Skin disease Skin eruption Survey Vaccination
As of October 2023, nearly 45,000,000 Koreans received at least one vaccine dose against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and nearly 34,000,000 Koreans were diagnosed at least once with coronavirus dis- ease 2019 (COVID-19)1. Although the global community rallied together to develop vaccines against SARS-CoV-2 following the emergence of the unprecedented COVID-19 pandemic, more than five million individuals across the globe died from the disease2. Furthermore, the urgent need for vaccines did not allow for a sufficient period of vaccine development, raising concerns regarding the efficacy and safety of the many newly developed vaccines and leading to vaccine refusal by some individuals. Nevertheless, concerted efforts by experts and government officials have led to the complete vaccination of a majority of the population, resulting in a subsiding trend in the COVID-19 pandemic.
Since the beginning of the global battle against the COVID-19 pandemic, numerous studies and case reports have documented the development of cutaneous reactions following SARS-CoV-2 vaccination or infection. Initial observations of cutaneous manifestations in vaccinated or infected patients have piqued the interest of researchers and clinicians alike. Previous studies have reported diverse dermatologic findings related to SARS-CoV-2 vaccination, including injection-site reactions, urticarial eruptions, papulosquamous eruptions, alopecia areata, and herpes zoster, among others3-7. The estimated incidence of cutaneous reactions following SARS-CoV-2 vaccination is 30%, albeit with a large variation among the studies6. Similarly, cutaneous manifestations of COVID-19 have been extensively documented, such as one study by Recalcati et al.8, which identified various cutaneous lesions, including erythematous rash, vesicular eruptions, urticaria, and chilblain-like lesions, in afflicted individuals9.
The majority of the large number of clinical studies elucidating the epidemiology of cutaneous manifestations of COVID-19 and those stemming from SARS-CoV-2 vaccination have been conducted in Western populations, and scholarly contributions originating from the Eastern Asian region are lacking from the current literature. The geographical imbalance in research output indicates a gap in our current understanding of the cutaneous reactions to SARS-CoV-2 infection and vaccination and highlights the need for the incorporation of the Eastern Asian perspective. Therefore, we conducted a questionnaire-based survey on the epidemiology and clinical characteristics of the cutaneous manifestations of SARS-CoV-2 infection and vaccination.
1. Study design
This multicenter, questionnaire-based survey was performed in 12 university hospitals across the Republic of Korea as follows: Asan Medical Center, Uijeongbu St. Mary's Hospital, Kyung Hee University Hospital, Daegu Catholic University Hospital, Jeonbuk National University Hospital, Konkuk University Hospital, Kyungpook National University Hospital, Inje University Busan Paik Hospital, National Medical Center, Chosun University Hospital, Pusan National University Yangsan Hospital, and Hanyang University Guri Hospital. Approval was obtained from the Institutional Review Boards of all participating hospitals before the survey.
2. Questionnaire-based survey
The study questionnaire included 35 and 42 questions related to cutaneous manifestations observed after SARS-CoV-2 infection and vaccination, respectively. The question- naire was administered to patients who visited the dermatology outpatient clinic of the participating hospitals. Informed consent was obtained from all survey participants. The survey was conducted between October 2022 and May 2023, and all responses were collected by July 31, 2023. Both the original Korean version and the English version of the questionnaire can be found in Supplementary Materials. After the collection of all responses, causality assessment was performed by a dermatologist, and the World Health Organization-Uppsala Monitoring Centre criteria were used for causality assessment based on the cutaneous lesions present at the time of the survey and the clinical information provided by respondents10.
3. Statistical Analysis
Data were presented as numbers and percentages of responders. Unanswered questionnaire items were excluded from the analysis. The χ2 or Fisher's exact test was used to compare categorical variables, and Student's t test was used to compare continuous variables. All statistical analyses were performed using IBM SPSS statistics version 22 (IBM, Armonk, NY, USA). A p value of <0.05 was considered statistically significant.
1. Cutaneous reactions to SARS-CoV-2 vaccin- ation
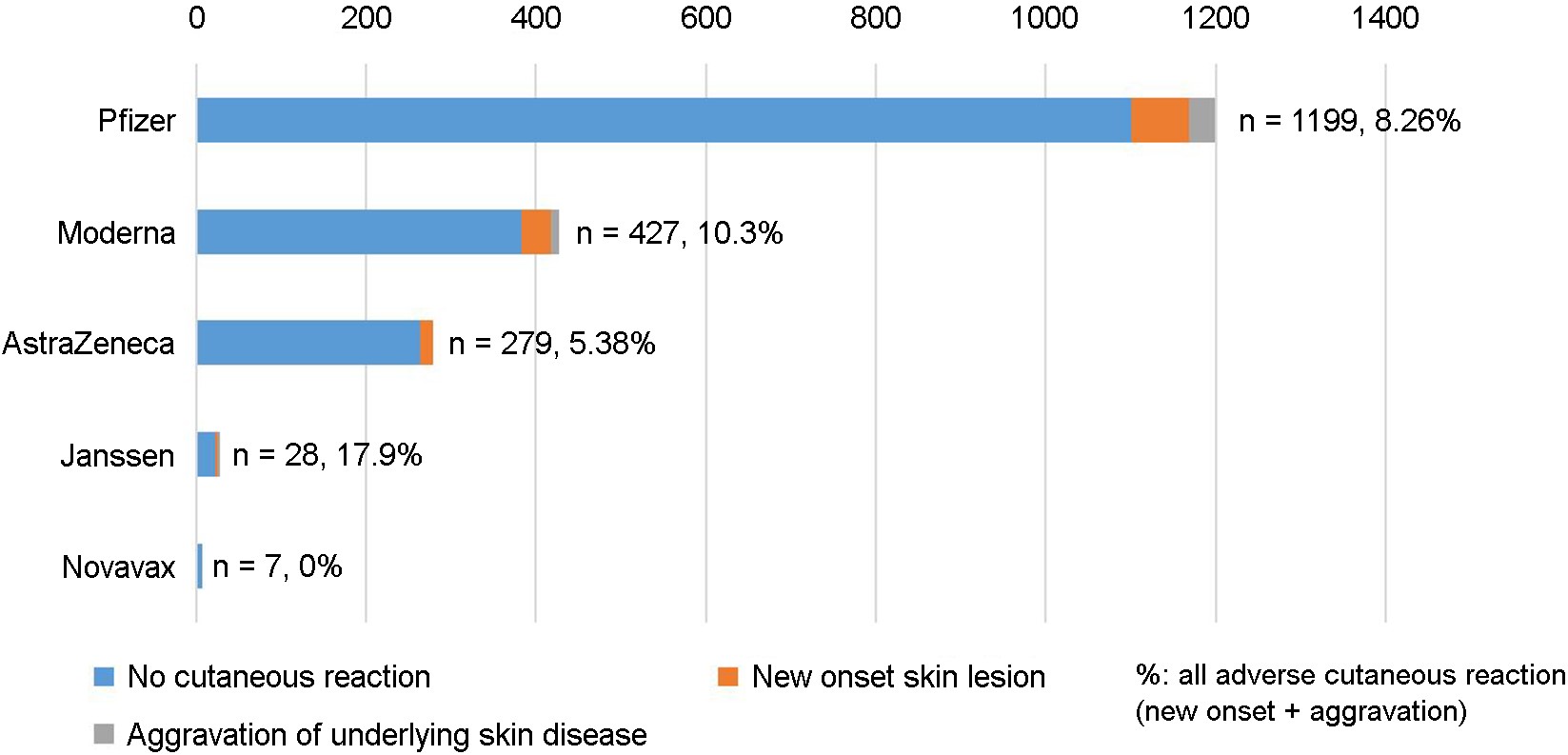
A total of 707 participants who received SARS-CoV-2 vaccinations completed the questionnaire. Five different SARS-CoV-2 vaccines (AstraZeneca, Janssen, Moderna, Novavax, and Pfizer-BioNTech) were used in Korea during the CVID-19 pandemic. Fig. 1 summarizes the proportions of respondents who developed cutaneous reactions to specific SARS-CoV-2 vaccines.
1) Demographic characteristics of participant and risk for cutaneous manifestations after SARS-CoV-2 vaccination
The demographic characteristics of participants categorized by the vaccine type are presented in Table 1. The mean respondent age was 44.26 years, with a male/female ratio of 1:1.1. Following SARS-CoV-2 vaccination, 20.23% and 5.94% of the respondents reported the onset of new cutaneous lesions or aggravation of preexisting cutaneous conditions, respectively. Most of the respondents were patients visiting dermatology outpatient clinics, as reflected in their diverse dermatologic conditions. The most common dermato- logic condition was atopic dermatitis, followed by psoriasis and hair loss, which included all types of hair loss diseases. The risk for the onset of new cutaneous lesions following SARS-CoV-2 vaccination varied from 10.71% (Janssen) to 0% (Novavax), albeit without statistically significant difference among the vaccines. Conversely, the risk for the aggravation of preexisting cutaneous conditions after SARS-CoV-2 vaccination was the lowest for the AstraZeneca vaccine (0%), which was significantly lower than that for the other vaccines (p = 0.018).
|
Characteristics |
Pfizer (n=381) |
Pfizer (n=414) |
Pfizer (n=322) |
Pfizer (n=82) |
AZ (n=160) |
AZ (n=103) |
AZ (n=16) |
Moderna (n=142) |
Moderna (n=157) |
Moderna (n=128) |
Janssen (n=23) |
Janssen (n=5) |
Novavax (n=1) |
Novavax
(2nd) (n=2) |
Novavax (n=4) |
Total (n=707) |
|
Sex |
||||||||||||||||
|
Male |
164 |
175 |
135 |
34 |
78 |
52 |
9 |
76 |
89 |
69 |
17 |
4 |
1 (100) |
1 |
1 |
336 |
|
Female |
217 |
229 |
187 |
48 |
82 |
51 |
7 |
66 |
68 |
59 |
6 |
1 (20) |
0 |
1 |
3 |
371 |
|
Age |
41.11 |
41.98 |
46.27 |
59.25 |
53.67 |
53.84 ±17.62 |
62.25 |
41.18 |
42.97 |
46.65 |
43.52 |
46.2 |
33 |
26 |
28.75 |
44.26 |
|
Past
medical |
||||||||||||||||
|
Hypertension |
42 |
50 |
40 |
22 |
25 |
12 |
4 |
24 |
28 |
26 |
4 |
1 |
0 |
0 |
0 |
111 |
|
Diabetes |
21 |
25 |
24 |
12 |
18 |
8 |
2 |
8 |
12 |
11 |
0 |
1 |
0 |
0 |
0 |
55 |
|
Respiratory |
3 |
4 |
7 |
2 |
7 |
7 |
0 |
8 |
7 |
7 |
1 |
0 |
0 |
1 |
1 |
20 |
|
Autoimmune |
5 |
5 |
5 |
3 |
3 |
2 |
0 |
4 |
4 |
3 |
0 |
0 |
0 |
0 |
0 |
14 |
|
Malignancy |
5 |
7 |
5 |
0 |
6 |
3 |
1 |
3 |
3 |
2 |
0 |
0 |
0 |
0 |
0 |
16 |
|
Other |
34 |
58 |
53 |
21 |
33 |
21 |
2 |
14 |
13 |
11 |
1 |
1 |
0 |
0 |
0 |
71 |
|
Past
dermatologic |
||||||||||||||||
|
Atopic |
24 |
38 |
18 |
5 |
6 |
2 |
0 |
10 |
10 |
8 |
1 |
1 |
0 |
0 |
0 |
60 |
|
Psoriasis |
34 |
36 |
29 |
6 |
9 |
6 |
4 |
8 |
10 |
7 |
4 |
1 |
0 |
0 |
0 |
57 |
|
Contact |
10 |
5 |
6 |
1 |
5 |
3 |
3 |
1 |
2 |
2 |
1 |
0 |
0 |
0 |
0 |
16 |
|
Urticaria |
10 |
7 |
6 |
1 |
3 |
1 |
1 |
1 |
4 |
1 |
2 |
0 |
0 |
0 |
0 |
17 |
|
Alopecia |
14 |
16 |
8 |
1 |
6 |
4 |
3 |
4 |
4 |
5 |
1 (4.35) |
0 |
0 |
0 |
0 |
29 |
|
Acne |
7 |
7 |
5 |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
|
Rosacea |
7 |
8 |
8 |
3 |
2 |
1 |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
10 (1.41) |
|
Other |
40 |
41 |
32 |
13 |
14 |
11 |
1 |
9 |
9 |
7 |
3 |
0 |
0 |
0 |
0 |
51 |
|
Systemic
symptom |
||||||||||||||||
|
Fever |
22 |
25 |
24 |
6 |
15 |
8 |
0 |
13 |
12 |
11 |
2 |
1 |
0 |
0 |
0 |
54 |
|
Chill |
9 |
12 |
11 (3.42) |
3 |
10 |
5 (4.85) |
0 |
8 |
8 |
5 |
1 |
0 |
0 |
0 |
0 |
30 |
|
Cough |
5 |
7 |
8 (2.48) |
2 |
3 |
3 |
0 |
6 |
3 |
3 |
1 |
1 |
0 |
0 |
0 |
15 |
|
Sore
throat |
13 |
15 |
11 |
3 (3.66) |
4 |
1 |
0 |
6 |
4 |
4 |
1 |
1 |
0 |
0 |
0 |
24 |
|
Myalgia |
25 |
29 |
23 |
5 |
24 |
12 (11.65) |
2 |
20 |
20 |
18 |
2 |
1 |
0 |
0 |
1 |
73 |
|
Other |
7 |
6 |
9 |
2 |
5 |
4 |
0 |
2 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
16 |
|
Skin
condition |
||||||||||||||||
|
New
onset skin |
20 |
16 |
24 |
8 |
9 |
4 |
2 |
4 |
14 |
17 |
2 |
1 |
0 |
0 |
0 |
143 |
|
Aggravation
of |
13 |
11 |
6 |
1 |
0 |
0 |
0 |
4 |
1 |
4 |
1 |
1 |
0 |
0 |
0 |
42 |
|
AZ, AstraZeneca; Values are presented as
absolute numbers only, mean ± standard deviation, or percentages (%) |
||||||||||||||||
2) Clinical characteristics of the respondents with new-onset cutaneous lesions after SARS-CoV-2 vaccination
The new-onset cutaneous lesions reported after SARS- CoV-2 vaccination were classified according to the vaccine product administered prior their development (Table 2). The types of the new-onset cutaneous lesions were not significantly different among the vaccine products. The most com- mon cutaneous lesion was urticaria/morbilliform eruption, which accounted for 61.86% of all new-onset cutaneous lesions, followed by eczema (46.61%), hair loss (22.88%), local injection-site reaction (16.10%), and psoriasiform erup- tion (11.02%). Pruritus was the most common subjective symptom of the cutaneous lesions, reported by 92.37% of the respondents, followed by heating sensation (44.92%). Pain and sensory changes were reported by 19.49% and 9.32% of the respondents, respectively. Approximately 30% of all cutaneous lesions developed after the first vaccination. The same percentage of cutaneous lesions developed after the second vaccination, whereas 36.44% of the cutaneous lesions developed following the third vaccination. While 60% of the respondents who received the AstraZeneca vaccine developed new cutaneous lesions after the first vaccination, new cutaneous reactions were noted more frequently after the second or third vaccination in respondents who received the Pfizer or Moderna vaccine. Only 10.17% of all new-onset cutaneous lesions developed either the fourth or the fifth vaccination. The distribution of new-onset cutaneous lesion types is shown in the Supplementary Table 1. Overall, more than half of the new-onset cutaneous lesions were located on arms, legs, and back.
|
Characteristics |
Pfizer (n=68) |
AZ (n=15) |
Moderna (n=39) |
Janssen (n=3) |
Total (n=118) |
|
Sex |
|||||
|
Male |
28 (45.9) |
6 (40) |
22 (56.41) |
2 (66.67) |
58 (49.15) |
|
Female |
33 (54.1) |
9 (60) |
17 (43.59) |
1 (33.33) |
60 (50.85) |
|
Age (years) |
48.06±16.94 |
47.96±15.93 |
44.27±16.96 |
45.33±17.03 |
46.77±17.58 |
|
Onset (days) |
47.05±73.76 |
13.00±19.73 |
40.90±54.93 |
3.5±1.09 |
39.81±51.50 |
|
Cutaneous reaction |
|||||
|
Urticaria and |
38 (62.3) |
10 (66.67) |
25 (64.1) |
0 (0) |
73 (61.86) |
|
Eczema |
30 (49.18) |
5 (33.33) |
18 (46.15) |
2 (66.67) |
55 (46.61) |
|
Vesicular eruption |
6 (9.84) |
3 (20) |
3 (7.69) |
0 (0) |
12 (10.17) |
|
Psoriasiform lesions |
9 (14.75) |
0 (0) |
3 (7.69) |
1 (33.33) |
13 (11.02) |
|
Acne and folliculitis |
6 (9.84) |
2 (13.33) |
3 (7.69) |
0 (0) |
11 (9.32) |
|
Hair loss |
15 (24.59) |
4 (26.67) |
6 (15.38) |
2 (66.67) |
27 (22.88) |
|
Hypopigmentation |
3 (4.92) |
1 (6.67) |
0 (0) |
0 (0) |
4 (3.39) |
|
Hyperpigmentation |
4 (6.56) |
1 (6.67) |
6 (15.38) |
0 (0) |
11 (9.32) |
|
Mass |
2 (3.28) |
0 (0) |
2 (5.13) |
0 (0) |
4 (3.39) |
|
Ulcer and necrosis |
1 (1.64) |
0 (0) |
1 (2.56) |
0 (0) |
2 (1.69) |
|
Herpes zoster |
1 (1.64) |
0 (0) |
4 (10.26) |
1 (33.33) |
6 (5.08) |
|
Local injection site reaction |
8 (13.11) |
5 (33.33) |
6 (15.38) |
0 (0) |
19 (16.1) |
|
Systemic symptoms |
|||||
|
Pruritus |
52 (85.25) |
7 (46.67) |
37 (94.87) |
3 (100) |
99 (83.90) |
|
Pain |
10 (16.39) |
2 (13.33) |
10 (25.64) |
1 (33.33) |
23 (19.49) |
|
Heating sensation |
29 (47.54) |
7 (46.67) |
17 (43.59) |
0 (0) |
53 (44.92) |
|
Sensory change |
5 (8.2) |
1 (6.67) |
5 (12.82) |
0 (0) |
11 (9.32) |
|
Timing of skin reaction |
|||||
|
After 1st vaccination |
20 (32.79) |
9 (60) |
4 (10.26) |
2 (66.67) |
35 (29.66) |
|
After 2nd vaccination |
16 (26.23) |
4 (26.67) |
14 (35.9) |
1 (33.33) |
35 (29.66) |
|
After 3rd vaccination |
24 (39.34) |
2 (13.33) |
17 (43.59) |
0 (0) |
43 (36.44) |
|
Others (4th and 5th) |
8 (13.11) |
0 (0) |
4 (10.26) |
0 (0) |
12 (10.17) |
|
AZ,
AstraZeneca; Values are presented as absolute numbers only, mean ± standard deviation, or percentages (%) |
|||||
3) Demographic characteristics and the clinical course of respondents with common new-onset cutaneous lesions after SARS-CoV-2 vaccination
The questionnaire included queries on the clinical course of cutaneous lesions. However, sufficient data for analysis were available only for the three most common conditions after the exclusion of the questions that were left blank by the respondents. Data on the clinical course and risk factors for the three most common cutaneous lesions (urticaria/ morbilliform rash, eczema, and hair loss) are summarized in Table 3. Briefly, the male/female ratio, age, and the time of onset did not significantly differ among the three most common cutaneous reactions. Respondents reported various systemic symptoms associated with vaccination, among which myalgia was the most common. Albeit exhibiting variation, the frequency of systemic symptoms did not significantly differ among the respondents with different cutaneous reactions. Fewer than 30% of the respondents who had urticaria/ morbilliform rash after vaccination received treatment. Con- versely, 52.73% and 65.22% of the respondents who developed eczema and hair loss after vaccination, respectively, received treatment. The majority of the respondents either visited outpatient clinics for treatment or waited for spontaneous improvement. Only two respondents were admitted. The final outcome varied among the cutaneous lesion types. The proportion of respondents who reported worsening of the cutaneous lesions over time was significantly higher for eczema than for urticaria/morbilliform rash or hair loss (23.64% vs. X% and y%, respectively; p = 0.040). In contrast, 14.16% of the respondents with urticaria/morbilliform rash reported the complete clearance of cutaneous lesions at some point, which was significantly higher than that reported for eczema and hair loss (X% and Y%, respectively; p = 0.033).
|
Characteristics |
Urticaria
& |
% |
Eczema |
% |
Hair
loss |
% |
p-value |
|
Sex |
0.326 |
||||||
|
Male |
65 |
52.00 |
33 |
60.00 |
11 |
47.83 |
|
|
Female |
60 |
48.00 |
22 |
40.00 |
12 |
52.17 |
|
|
Age |
50.62±18.35 |
|
50.57±17.60 |
|
49.39±17.62 |
|
0.587 |
|
Time
from vaccination |
44.63±51.09 |
|
37.11±51.25 |
|
24.36±20.18 |
|
0.389 |
|
Systemic symptoms |
|||||||
|
Fever |
35 |
28.00 |
18 |
32.73 |
7 |
30.43 |
0.722 |
|
Chill |
16 |
12.80 |
8 |
14.55 |
3 |
13.04 |
0.956 |
|
Cough |
10 |
8.00 |
6 |
10.91 |
2 |
8.70 |
0.653 |
|
Sore throat |
20 |
16.00 |
11 |
20.00 |
5 |
21.74 |
0.941 |
|
Myalgia |
47 |
37.60 |
27 |
49.09 |
12 |
52.17 |
0.484 |
|
Oral medication(s) to |
|||||||
|
Antipyretics |
13 |
30.23 |
13 |
27.08 |
4 |
26.67 |
|
|
Systemic corticosteroid |
11 |
25.58 |
10 |
20.83 |
6 |
40.00 |
|
|
Systemic antiviral agent |
1 |
2.33 |
0 |
0.00 |
0 |
0.00 |
|
|
Systemic antibiotics |
4 |
9.30 |
5 |
10.42 |
1 |
6.67 |
|
|
Systemic antihistamines |
14 |
32.56 |
20 |
41.67 |
4 |
26.67 |
|
|
Systemic |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
|
|
Dermatologic symptoms |
|||||||
|
Pruritus |
94 |
75.20 |
53 |
96.36 |
11 |
47.83 |
|
|
Pain |
20 |
16.00 |
15 |
27.27 |
3 |
13.04 |
|
|
Heating sensation |
48 |
38.40 |
27 |
49.09 |
6 |
26.09 |
|
|
Sensory change |
9 |
7.20 |
7 |
12.73 |
1 |
4.35 |
|
|
Treatment for |
|||||||
|
Yes |
27 |
21.60 |
29 |
52.73 |
15 |
65.22 |
|
|
No |
98 |
78.40 |
26 |
47.27 |
8 |
34.78 |
|
|
Mode of treatment |
|||||||
|
Observation at home (with |
12 |
30.77 |
14 |
25.45 |
3 |
13.04 |
|
|
Outpatient visit |
26 |
66.67 |
26 |
47.27 |
20 |
86.96 |
|
|
Admission |
1 |
2.56 |
1 |
1.82 |
0 |
0.00 |
|
|
ICU care |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
|
|
Final clinical outcome |
|||||||
|
Persistent |
57 |
50.44 |
24 |
43.64 |
11 |
47.83 |
0.898 |
|
Aggravation |
14 |
12.39 |
13 |
23.64 |
2 |
8.70 |
0.04* |
|
Partial remission |
26 |
23.01 |
13 |
23.64 |
5 |
21.74 |
0.94 |
|
Complete remission |
16 |
14.16 |
2 |
3.64 |
1 |
4.35 |
0.033* |
|
Refused next vaccination |
52 |
41.60 |
27 |
49.09 |
11 |
47.83 |
0.907 |
|
Presence of corresponding |
29 |
23.20 |
49 |
89.09 |
21 |
91.30 |
|
|
Causality assessment by |
|||||||
|
Certain |
2 |
1.72 |
2 |
3.64 |
0 |
0.00 |
|
|
Probable |
13 |
11.21 |
4 |
7.27 |
3 |
13.04 |
|
|
Possible |
56 |
48.28 |
24 |
43.64 |
12 |
52.17 |
|
|
Less likely |
13 |
11.21 |
4 |
7.27 |
1 |
4.35 |
|
|
Limited evaluation |
13 |
11.21 |
4 |
7.27 |
2 |
8.70 |
|
|
Unable to assess |
19 |
16.38 |
14 |
25.45 |
4 |
17.39 |
|
|
Values
are presented as absolute numbers only, mean ± standard deviation, or percentages (%); *p < 0.05 |
|||||||
4) Causality assessment of new-onset cuta- neous lesions after SARS-CoV-2 vaccination
Based on the cutaneous lesion type and the clinical in- formation including the time of onset, causality assessment was performed for each case by dermatologists. Only 9.3% (18 out of 194) of all cases were determined as unlikely to be caused by SARS-CoV-2 vaccination. Causality assessment could not be performed in 28.9% (56 out of 194) of the cases. The majority of the remaining cases were assessed as possibly caused by SARS-CoV-2 vaccination.
5) Demographic characteristics of respond- ents who reported aggravation of preexisting cutaneous conditions after SARS-CoV-2 vaccination
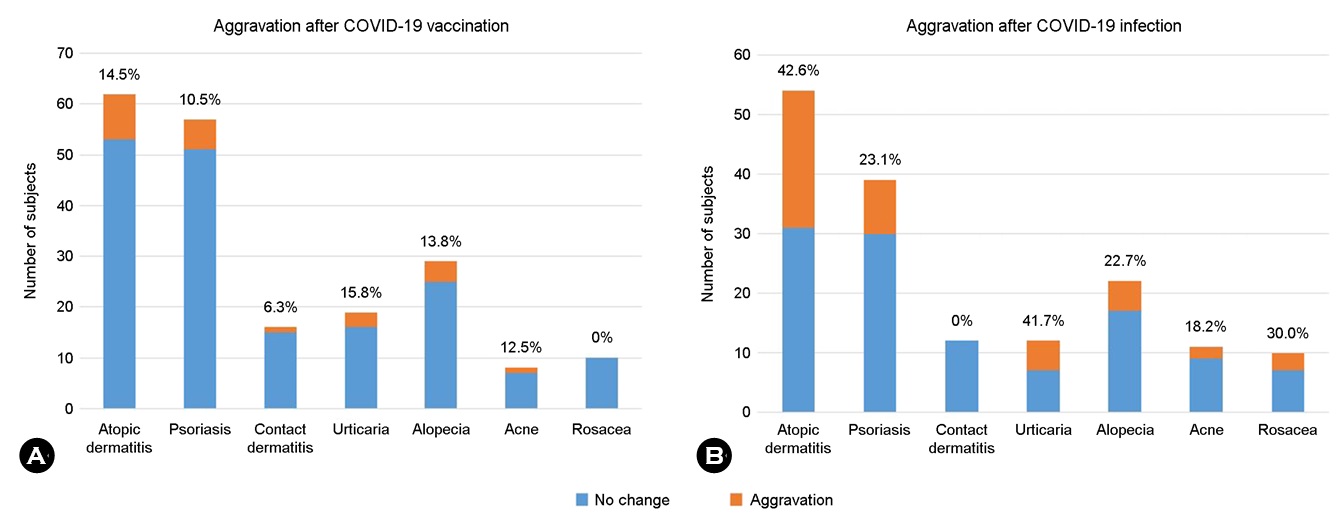
Aggravation of the preexisting cutaneous conditions was reported by 42 (5.94%) respondents, whose demographic characteristics are summarized in Table 4. Briefly, the male /female ratio was 1:1.63. None of the respondents who received the AstraZeneca vaccine reported aggravation of preexisting cutaneous conditions. There was no significant difference in the proportion of specific preexisting cutaneous diseases that exhibited aggravation among the different vaccines. Atopic dermatitis (21.43%) was the most commonly reported preexisting cutaneous condition that aggravated following vaccination. Although systemic symptoms was reported by respondents who experienced aggravation of preexisting cutaneous conditions after vaccination, the rates were low, below 8% for all. Across all vaccine products, 42.86% of the respondents experienced aggravation of cutaneous lesions after the first vaccination, with a consistently decline in the frequency of aggravation with each booster shot. Fig. 2A illustrates the proportions of respondents who reported aggravation of specific preexisting cutaneous conditions. The three most common preexisting cutaneous conditions were atopic dermatitis, psoriasis, and alopecia, which were reported to be aggravated after SARS-CoV-2 vaccination by comparable proportions of respondents. Interestingly, no respondent with rosacea reported aggravation.
|
Characteristics |
Pfizer (n=31) |
% |
AZ (n=0) |
% |
Moderna (n=9) |
% |
Janssen (n=2) |
% |
Total (n=42) |
% |
|
Sex |
||||||||||
|
Male |
12 |
38.71 |
0 |
0.00 |
2 |
22.22 |
2 |
100.00 |
16 |
38.10 |
|
Female |
19 |
61.29 |
0 |
0.00 |
7 |
77.78 |
0 |
0.00 |
26 |
61.90 |
|
Age (years) |
42.56 |
|
N/A |
|
51.56 |
|
37 |
|
45.02 |
|
|
Underlying skin disease |
||||||||||
|
Atopic dermatitis |
7 |
22.58 |
0 |
0.00 |
0 |
0.00 |
2 |
100.00 |
9 |
21.43 |
|
Psoriasis |
5 |
16.13 |
0 |
0.00 |
1 |
11.11 |
0 |
0.00 |
6 |
14.29 |
|
Acne and folliculitis |
1 |
3.23 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
1 |
2.38 |
|
Contact dermatitis |
1 |
3.23 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
1 |
2.38 |
|
Urticaria |
1 |
3.23 |
0 |
0.00 |
2 |
22.22 |
0 |
0.00 |
3 |
7.14 |
|
Hair loss |
3 |
9.68 |
0 |
0.00 |
1 |
11.11 |
0 |
0.00 |
4 |
9.52 |
|
Vitiligo |
1 |
3.23 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
1 |
2.38 |
|
Other / No response |
13 |
41.94 |
0 |
0.00 |
5 |
55.56 |
0 |
0.00 |
18 |
42.86 |
|
Systemic symptoms |
||||||||||
|
Fever |
2 |
6.45 |
0 |
0.00 |
1 |
11.11 |
0 |
0.00 |
3 |
7.14 |
|
Chill |
1 |
3.23 |
0 |
0.00 |
1 |
11.11 |
0 |
0.00 |
2 |
4.76 |
|
Cough |
1 |
3.23 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
1 |
2.38 |
|
Sore throat |
2 |
6.45 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
2 |
4.76 |
|
Myalgia |
1 |
3.23 |
0 |
0.00 |
1 |
11.11 |
1 |
50.00 |
3 |
7.14 |
|
Timing of skin reaction |
||||||||||
|
After 1st vaccination |
13 |
41.94 |
0 |
0.00 |
4 |
44.44 |
1 |
50.00 |
18 |
42.86 |
|
After 2nd vaccination |
11 |
35.48 |
0 |
0.00 |
1 |
11.11 |
1 |
50.00 |
13 |
30.95 |
|
After 3rd vaccination |
6 |
19.35 |
0 |
0.00 |
4 |
44.44 |
0 |
0.00 |
10 |
23.81 |
|
Others (4th and 5th) |
1 |
3.23 |
0 |
0.00 |
0 |
0.00 |
0 |
0.00 |
1 |
2.38 |
|
AZ,
AstraZeneca; Values are presented as absolute numbers only, mean ± standard deviation, or
percentages (%) |
||||||||||
6) Risk factors for the new onset and aggra- vation of preexisting cutaneous lesions after SARS-CoV-2 vaccination
We assessed the potential risk factors for the development of new cutaneous lesions and the aggravation of preexisting cutaneous lesions after SARS-CoV-2 vaccination (Table 5). The respondents who developed new cutaneous lesions after SARS-CoV-2 vaccination were significantly older than those who did not experience any cutaneous reactions (43.29 ± 17.63 vs. 48.51 ± 16.97 years, p = 0.001). The presence of underlying disease, including malignancy, hypertension, diabetes mellitus, respiratory disease, and autoimmune disease, was not associated with a higher risk for the onset or aggravation of cutaneous lesions after SARS-CoV-2 vaccination. Myalgia was the only systemic symptom following SARS-CoV-2 vaccination that was significantly associated with a higher risk for the aggravation of preexisting cutaneous conditions (odds ratio [OR] 45.33, 95% confidence interval [CI] 3.16~650.97, p = 0.011). However, the presence of systemic symptoms associated with SARS-CoV-2 vaccination was strongly associated with a higher risk for the new onset of cutaneous lesions; the observed increased risk was present for all five reported symptoms (fever: OR 13.03, 95% CI 4.74~48.92, p < 0.001; chill: OR 5.31, 95% CI 1.93~20.45, p = 0.005; cough: OR 7.34, 95% CI 1.50~11.53, p = 0.038; sore throat: OR 13.39, 95% CI 1.77~101.55, p = 0.002; and myalgia: OR 22.34, 95% CI 8.77~83.75, p < 0.001) (Fig. 3A).

|
No cutaneous |
% |
New-onset |
% |
p-value |
Aggravation of |
% |
p-value |
|
|
Sex |
||||||||
|
M |
296 |
47.06 |
58 |
49.15 |
0.639 |
16 |
38.10 |
0.425 |
|
F |
333 |
52.94 |
60 |
50.85 |
26 |
61.90 |
||
|
Age |
43.29 |
|
48.51 |
|
0.001 |
45.02 |
|
0.269 |
|
Underlying disease |
||||||||
|
Malignancy |
||||||||
|
Yes |
13 |
2.18 |
3 |
2.61 |
1 |
2 |
4.76 |
0.21 |
|
No |
583 |
97.82 |
112 |
97.39 |
40 |
95.24 |
||
|
HTN |
0.00 |
|||||||
|
Yes |
77 |
12.92 |
23 |
20.00 |
0.077 |
8 |
19.05 |
0.343 |
|
No |
519 |
87.08 |
92 |
80.00 |
34 |
80.95 |
||
|
DM |
0.00 |
|||||||
|
Yes |
43 |
7.21 |
8 |
6.96 |
1 |
2 |
4.76 |
0.759 |
|
No |
553 |
92.79 |
107 |
93.04 |
40 |
95.24 |
||
|
Respiratory disease |
0.00 |
|||||||
|
Yes |
13 |
2.18 |
5 |
4.35 |
0.362 |
2 |
4.76 |
0.26 |
|
No |
583 |
97.82 |
110 |
95.65 |
40 |
95.24 |
||
|
Autoimmune disease |
0.00 |
|||||||
|
Yes |
9 |
1.51 |
3 |
2.61 |
0.709 |
2 |
4.76 |
0.16 |
|
No |
587 |
98.49 |
112 |
97.39 |
40 |
95.24 |
||
|
Systemic symptom |
||||||||
|
Fever |
||||||||
|
Yes |
3 |
4.23 |
50 |
36.50 |
<
0.001 |
1 |
33.33 |
0.156 |
|
No |
68 |
95.77 |
87 |
63.50 |
2 |
66.67 |
||
|
Chill |
||||||||
|
Yes |
3 |
4.23 |
26 |
18.84 |
0.005 |
1 |
33.33 |
0.156 |
|
No |
68 |
95.77 |
112 |
81.16 |
2 |
66.67 |
||
|
Cough |
||||||||
|
Yes |
1 |
1.41 |
13 |
9.42 |
0.038 |
1 |
33.33 |
0.08 |
|
No |
70 |
98.59 |
125 |
90.58 |
2 |
66.67 |
||
|
Sore throat |
||||||||
|
Yes |
1 |
1.41 |
23 |
16.67 |
0.002 |
0 |
0.00 |
1 |
|
No |
70 |
98.59 |
115 |
83.33 |
3 |
100.00 |
2. Cutaneous complications of SARS-CoV-2 infection
1) Demographic and clinical characteristics of respondents who experienced cutaneous complications after SARS-CoV-2 infection
A total of 682 participants who were infected with SARS-CoV-2 completed the questionnaire. Table 6 shows the demographic and clinical characteristics of all respondents. Among these 682 respondents, 91 (13.3%) and 66 (9.7%) reported the development of new cutaneous lesions and the aggravation of preexisting cutaneous conditions, respectively, after COVID-19 diagnosis. Urticaria/morbilliform rash was the most common new-onset cutaneous lesion (72.53%), followed by hair loss (23.08%), vesicular eruption (15.38%), acne and folliculitis (13.19%), and eczema (9.89%). Only one respondent reported chilblain-like eruptions in acral regions. Among all respondents who reported aggravation of preexisting cutaneous lesions after SARS-CoV-2 infection, atopic dermatitis was the most common condition (34.85%), followed by psoriasis (13.64%), hair loss (7.58%), and urticaria (7.58%). Fig. 2B shows the number of respondents with specific cutaneous lesions and the proportion of those who reported aggravation after SARS-CoV-2 infection. Pruritus was the most common symptom of cutaneous complications. Respondents who developed new cutaneous lesions following COVID-19 were significantly older than those without new cutaneous lesions (p = 0.046). Systemic COVID-19 symptoms were more common in respondents who developed new cutaneous lesions than in those without new cutaneous lesions (fever: OR 7.49, 95% CI 3.93~14.29, p < 0.001; chill: OR 4.23, 95% CI 2.16~8.27, p < 0.001; cough: OR 6.42, 95% CI 3.40~12.12, p < 0.001; sore throat: OR 6.62, 95% CI 3.48~12.62, p < 0.001; and myalgia: OR 6.06, 95% CI 3.19~11.51, p < 0.001) (Fig. 3B). Moreover, the proportion of respondents with underlying autoimmune diseases was significantly higher in those who developed COVID-19-associated new-onset new cutaneous lesions than in those without new cutaneous lesions (OR 3.88; 95% CI, 1.22~12.31; p = 0.038). Other underlying diseases, including hypertension, diabetes mellitus, respiratory diseases, and malignancy, were not associated with an increased risk for the development of new cutaneous lesions. Neither underlying diseases nor systemic COVID-19 symptoms were associated with a higher risk for the aggravation of preexisting cutaneous lesions. Information on the final outcome of the new-onset cutaneous lesions following COVID-19 infection was avail- able in 13 respondents. The cutaneous lesions persisted in 5 (38.5%) respondents, aggravated over time in 3 (23.1%) respondents, and exhibited partial and complete resolution in 3 (23.1%) and 2 (15.4%) respondents, respectively.
|
Characteristics |
No skin (n=525) |
% |
New-onset (n=91) |
% |
p-value |
Aggravation of (n=66) |
% |
p-value |
Total (n=682) |
% |
|
Sex |
||||||||||
|
Male |
229 |
43.62 |
32 |
35.16 |
0.131 |
25 |
37.88 |
0.412 |
286 |
41.94 |
|
Female |
296 |
56.38 |
59 |
64.84 |
41 |
62.12 |
396 |
58.06 |
||
|
Age (years) |
39.57 ±15.95 |
|
43.43 ±15.90 |
|
0.046* |
39.54 ±15.97 |
|
0.907 |
40.14 |
|
|
Past medical history |
||||||||||
|
Hypertension |
39 |
7.43 |
13 |
14.29 |
0.212 |
3 |
4.55 |
0.095 |
55 |
8.06 |
|
Diabetes mellitus |
20 |
3.81 |
6 |
6.59 |
0.814 |
1 |
1.52 |
0.408 |
27 |
3.96 |
|
Respiratory disease |
9 |
1.71 |
5 |
5.49 |
0.071 |
2 |
3.03 |
0.996 |
16 |
2.35 |
|
Autoimmune disease |
6 |
1.14 |
4 |
4.40 |
0.014* |
2 |
3.03 |
0.155 |
12 |
1.76 |
|
Malignancy |
8 |
1.52 |
2 |
2.20 |
0.923 |
3 |
4.55 |
0.915 |
13 |
1.91 |
|
Other |
29 |
5.52 |
20 |
21.98 |
2 |
3.03 |
51 |
7.48 |
||
|
New onset skin lesions |
0.00 |
|||||||||
|
Urticaria and |
N/A |
N/A |
66 |
72.53 |
|
7 |
10.61 |
|
N/A |
N/A |
|
Vesicular eruption |
N/A |
N/A |
14 |
15.38 |
N/A |
N/A |
N/A |
N/A |
||
|
Eczema |
N/A |
N/A |
9 |
9.89 |
N/A |
N/A |
N/A |
N/A |
||
|
Chilblain-like change |
N/A |
N/A |
1 |
1.10 |
|
N/A |
N/A |
|
N/A |
N/A |
|
Vasculitis |
N/A |
N/A |
2 |
2.20 |
N/A |
N/A |
N/A |
N/A |
||
|
Acne and folliculitis |
N/A |
N/A |
12 |
13.19 |
N/A |
N/A |
N/A |
N/A |
||
|
Hair loss |
N/A |
N/A |
21 |
23.08 |
N/A |
N/A |
N/A |
N/A |
||
|
Hypopigmentation |
N/A |
N/A |
1 |
1.10 |
N/A |
N/A |
N/A |
N/A |
||
|
Hyperpigmentaion |
N/A |
N/A |
7 |
7.69 |
N/A |
N/A |
N/A |
N/A |
||
|
Ulcer and necrosis |
N/A |
N/A |
1 |
1.10 |
N/A |
N/A |
N/A |
N/A |
||
|
Symptoms without |
N/A |
N/A |
4 |
4.40 |
|
N/A |
N/A |
|
N/A |
N/A |
|
Aggravated underlying |
||||||||||
|
Atopic dermatitis |
N/A |
N/A |
N/A |
N/A |
23 |
34.85 |
N/A |
N/A |
||
|
Psoriasis |
N/A |
N/A |
N/A |
N/A |
9 |
13.64 |
N/A |
N/A |
||
|
Hair loss |
N/A |
N/A |
N/A |
N/A |
|
5 |
7.58 |
|
N/A |
N/A |
|
Acne and folliculitis |
N/A |
N/A |
N/A |
N/A |
|
2 |
3.03 |
|
N/A |
N/A |
|
Rosacea |
N/A |
N/A |
N/A |
N/A |
|
3 |
4.55 |
|
N/A |
N/A |
|
Urticaria |
N/A |
N/A |
N/A |
N/A |
|
5 |
7.58 |
|
N/A |
N/A |
|
Others |
N/A |
N/A |
N/A |
N/A |
|
10 |
15.15 |
|
N/A |
N/A |
|
Symptoms of skin lesion |
|
|
|
|
|
|
|
|
|
|
|
Pruritus |
N/A |
N/A |
69 |
75.82 |
|
57 |
86.36 |
|
N/A |
N/A |
|
Pain |
N/A |
N/A |
15 |
16.48 |
|
11 |
16.67 |
|
N/A |
N/A |
|
Heating sensation |
N/A |
N/A |
27 |
29.67 |
|
35 |
53.03 |
|
N/A |
N/A |
|
Sensory change |
N/A |
N/A |
1 |
1.10 |
|
4 |
6.06 |
|
N/A |
N/A |
|
Systemic symptoms |
|
|
|
|
|
|
|
|
|
|
|
Fever |
21 |
21.88 |
59 |
64.84 |
< 0.001* |
11 |
22.92 |
0.887 |
91 |
38.72 |
|
Chil |
16 |
16.67 |
44 |
48.35 |
< 0.001* |
9 |
18.75 |
0.756 |
69 |
29.36 |
|
Cough |
22 |
22.92 |
63 |
69.23 |
< 0.001* |
16 |
33.33 |
0.414 |
101 |
42.98 |
|
Sore throat |
20 |
20.83 |
61 |
67.03 |
< 0.001* |
9 |
18.75 |
0.365 |
90 |
38.30 |
|
Myalgia |
20 |
20.83 |
59 |
64.84 |
< 0.001* |
12 |
25.00 |
1 |
91 |
38.72 |
|
Mode of COVID-19 |
|
|
|
|
|
|
|
|
|
|
|
PCR |
347 |
69.26 |
50 |
54.95 |
|
37 |
56.06 |
|
434 |
63.64 |
|
Rapid antigen test |
120 |
23.95 |
33 |
36.26 |
|
17 |
25.76 |
|
170 |
24.93 |
|
Self-test |
34 |
6.79 |
6 |
6.59 |
|
6 |
9.09 |
|
46 |
6.74 |
|
Values are presented as
absolute numbers only, mean ± standard deviation, or percentages (%), *p < 0.05 |
||||||||||
2) Clinical characteristics of respondents who experienced cutaneous complications of SARS-CoV-2 infection
Table 7 shows the clinical characteristics of respondents who developed the three most commonly reported new-onset cutaneous lesions and the risk factor analysis. Similar to that observed with the risk assessment for cutaneous reactions to SARS-CoV-2 vaccination, all three common cutaneous reactions (urticaria/morbilliform rash, vesicular eruption, and hair loss) were associated with the presence of systemic COVID-19 symptoms. Moreover, the mean age of the respondents who developed urticaria/morbilliform rash was significantly higher than that of those who did not experience any cutaneous complications (39.57 ± 15.95 vs. 45.76 ± 15.96 years, p = 0.004). The anatomical distribution of the new-onset cutaneous lesions is presented in Supplementary Table 2.
|
Characteristics |
No skin |
% |
Urticaria & |
% |
p-value |
Vesicular |
% |
p-value |
Hair loss |
% |
p-value |
|
Sex |
|||||||||||
|
Male |
229 |
43.62 |
23 |
18.40 |
8 |
57.14 |
7 |
33.33 |
|||
|
Female |
296 |
56.38 |
43 |
34.40 |
6 |
42.86 |
14 |
66.67 |
|||
|
Age |
39.57 |
|
45.76 |
|
0.004* |
45.57 |
|
0.155 |
49.39 |
|
0.398 |
|
Systemic symptoms |
|||||||||||
|
Fever |
21 |
21.88 |
41 |
32.80 |
< 0.001* |
9 |
64.29 |
0.002* |
13 |
61.90 |
< 0.001* |
|
Chill |
16 |
16.67 |
27 |
21.60 |
0.001* |
6 |
42.86 |
0.033* |
11 |
52.38 |
< 0.001* |
|
Cough |
22 |
22.92 |
39 |
31.20 |
< 0.001* |
10 |
71.43 |
0.001* |
10 |
47.62 |
0.012* |
|
Sore throat |
20 |
20.83 |
39 |
31.20 |
< 0.001* |
11 |
78.57 |
< 0.001* |
13 |
61.90 |
< 0.001* |
|
Myalgia |
20 |
20.83 |
42 |
33.60 |
< 0.001* |
9 |
64.29 |
0.002* |
11 |
52.38 |
0.002* |
|
Dermatologic symptoms |
|||||||||||
|
Pruritus |
N/A |
N/A |
57 |
45.60 |
12 |
85.71 |
13 |
61.90 |
|||
|
Pain |
N/A |
N/A |
23 |
18.40 |
2 |
14.29 |
0 |
0.00 |
|||
|
Heating sensation |
N/A |
N/A |
26 |
20.80 |
6 |
42.86 |
4 |
19.05 |
|||
|
Sensory change |
N/A |
N/A |
1 |
0.80 |
0 |
0.00 |
0 |
0.00 |
|||
|
Presence of corresponding |
|||||||||||
|
Yes |
N/A |
N/A |
44 |
81.48 |
10 |
90.91 |
16 |
76.19 |
|||
|
No |
N/A |
N/A |
10 |
18.52 |
1 |
9.09 |
5 |
23.81 |
|||
|
Causality assessment |
|||||||||||
|
Certain |
N/A |
N/A |
1 |
1.85 |
0 |
0.00 |
1 |
4.76 |
|||
|
Probable |
N/A |
N/A |
2 |
3.70 |
0 |
0.00 |
3 |
14.29 |
|||
|
Possible |
N/A |
N/A |
24 |
44.44 |
6 |
54.55 |
9 |
42.86 |
|||
|
Unable to assess |
N/A |
N/A |
27 |
50.00 |
5 |
45.45 |
8 |
38.10 |
|||
|
Values are presented as absolute numbers only, mean ± standard deviation, or
percentages (%), p-value: compared
to the group without skin reaction, *p
< 0.05 |
|||||||||||
In the present questionnaire-based survey study, which aimed to investigate the epidemiology and clinical characteristics of cutaneous reactions following SARS-CoV-2 infection and vaccination in the Republic of Korea, we found that 20.23% of the respondents reported cutaneous reactions after SARS-CoV-2 vaccination. Urticaria/morbilliform rash was the most common manifestation and often accompanied by pruritus. In general, new-onset cutaneous reactions occurred at a similar rate (approximately 30%) for the first three vaccine administrations. Interestingly, the risk for cutaneous reactions was significantly higher for the first dose of the AstraZeneca vaccine than for the other four vaccines. A similar trend was observed in previous studies investigating all adverse reactions, not confined to the cutaneous reactions11,12. This discrepancy might be due to the difference in immunogenicity among the vaccine categories (viral vector vs. mRNA). Notably, only 10.17% of the cutaneous lesions developed after the fourth or fifth vaccination, indicating a decreasing risk with subsequent booster shots. The development of new-onset cutaneous lesions after SARS-CoV-2 vaccination was significantly associated with older age and the presence of vaccination-related systemic symptoms. In contrast, cutaneous complications following SARS-CoV-2 infection were observed in 13.3% of the respondents and urticaria/ morbilliform rash was the most common presentation. The presence of systemic symptoms, such as fever, chill, cough, sore throat, and myalgia, was significantly associated with the development of new cutaneous lesions after infection. Furthermore, respondents who developed cutaneous complications after SARS-CoV-2 infection were significantly older than those without cutaneous complications.
The prevalence of cutaneous adverse effects following SARS-CoV-2 vaccination varies depending on the specific vaccine and population as well as the study design. The vast majority of previous studies reported the relative incidence of cutaneous side effects for different vaccine brands. However, Lacey et al. reported that cutaneous reactions subsequent to the administration of mRNA SARS-CoV-2 vaccines (Pfizer-BioNTech and Moderna) occurred in 1.9% of the vaccine recipients, which is considerably higher than that observed in the present study (6.33%)13. In the present study, we aimed to minimize the impact of selection bias by randomly distributing the questionnaire to patients in the participating clinics. Consequently, the rates of cutaneous complications resulting from SARS-CoV-2 vaccination observed in the present study should be considered a reliable estimate of the overall incidence of such cutaneous reactions. However, the discrepancy between the incidence of cutaneous reactions observed in the present study and that reported in previous studies may also stem from the different recording methods. In previous studies, physicians documented the cutaneous reactions, whereas the present study relied on respondents and involved more subjective assessment. Therefore, respondents might have attributed most of the cutaneous conditions to the SARS-CoV-2 vaccination, even in cases where the vaccines might not have been the cause. The accurate determination of the prevalence of SARS-CoV-2 vaccine-related cutaneous side effects requires larger-scale, cohort studies with the objective evaluation of cutaneous reactions by medical professionals, ideally dermatologists.
The association between age and the development of adverse reactions to SARS-CoV-2 vaccination has been demonstrated in previous studies14-16, which reported an increased risk for adverse reactions in younger individuals. However, these studies included only injection-site reactions as adverse cutaneous reactions. Our analyses indicating a higher risk for adverse cutaneous reactions following both SARS-CoV-2 vaccination and injection in older respondents suggest differences in the underlying pathologic mechanism between systemic reactions, such as fever, fatigue, myalgia, nausea, and vomiting, and cutaneous reactions. Systemic reactions are caused by systemic innate immune response17, whereas cutaneous vaccine reactions are classically considered a result of allergic response involving type I or type IV hyper-sensitivity reaction. Following the recent emergence of mRNA-based vaccines and the heightened public interest in vaccine-related adverse events, other potential mechanisms, including viral reactivation, molecular mimicry, antibody production, and immune complex deposition, have been proposed for cutaneous reactions18-20. Thus, the differential effect of age on vaccine-induced adverse reactions are likely due to mechanistic differences in reaction types.
The reported incidence of cutaneous manifestations of COVID-19 range from 0.19% to 20.45%8,21,22. In the present study, 13.3% of the respondents diagnosed with COVID-19 experienced cutaneous manifestations, which is likely a good estimate of the actual proportion of patients with COVID-19-related skin manifestations given that the data were based on over 600 respondents. Moreover, the questionnaire covered a broad spectrum of cutaneous conditions ranging from mild to severe forms and offered a more accurate estimate compared to prior clinical studies, which might have potentially overlooked mild skin reactions that might not be readily discernible to the evaluator. However, similar to the incidence of SARS-CoV-2 vaccination-related cutaneous reactions, the present study design might have overestimated the incidence of cutaneous manifestation of SARS-CoV-2 infection by including cutaneous conditions irrelevant to SARS-CoV-2 infection. Previous studies, mainly from North America and Europe, have indicated chilblain-like lesions as a main cutaneous reaction category in COVID-19, with an incidence ranging from 19% to 75%8,23-25. However, no respondent in the present study reported chilblain-like lesions. In fact, the reported incidence of such cutaneous lesions is lower than 1% in studies from Asian populations22,26. There- fore, our findings highlight the difference in the type of cutaneous reactions to COVID-19 between different ethnic groups.
To our knowledge, this is the first study reporting that the likelihood of developing cutaneous reactions was significantly higher in respondents who experienced systemic symptoms, such as fever, chill, myalgia, sore throat, and myalgia, after SARS-CoV-2 vaccination or infection. Although the temporal relationship between the onset of systemic symptoms and cutaneous reactions could not be evaluated, our findings highlight the importance of vigilant monitoring for cutaneous reactions in patients who develop systemic symptoms after SARS-CoV-2 vaccination or infection. Furthermore, the association between systemic symptoms and cutaneous reactions suggest that the activation of innate immune response (systemic symptoms) might trigger subsequent hypersensitivity reaction, molecular mimicry, or autoimmune reaction, resulting in cutaneous manifestations. Our findings suggest the presence of a complex interplay of the factors contributing to cutaneous reactions after infection, including immune response and systemic disease manifestations. However, detailed cellular and molecular analyses are warranted to confirm this immunologic connection.
The limitations of our study should be acknowledged. First, the study included individuals seeking care at university hospitals and, therefore, may not be a full representation of the general population of the Republic of Korea. Furthermore, the classification of cutaneous lesions might not be accurate as their classification was solely based on the participants' response. To aid the participants, color images of typical cutaneous lesions were offered for each category. Although the lesion categories were relatively straightforward, mostly consisting of common cutaneous lesions, accurate assessment of the lesions might have been challenging for the participants, especially in cases where the lesions might have completely cleared at the time of the survey. Similarly, the medical history of the participants might not be fully accurate because the medical records of individual participants were not reviewed. The relatively small number of respondents who received certain vaccine products is another main study limitation. Finally, the risk of recall bias was present due to the study design.
In conclusion, this study offers valuable data on the pre- valence, clinical characteristics, and risk factors for various cutaneous reactions observed in association with SARS-CoV-2 vaccination and infection, thereby contributing to our comprehensive understanding of the dermatologic impact of SARS-CoV-2.
References
1. Agency KDCaP. Coronavirus Disease 19 (COVID-19). 2023 [Cited 2023 October 16]. Available from: https:// ncov.kdca.go.kr/en
2. World Health Organization (WHO), A comprehensive view of global deaths directly and indirectly associated with the COVID-19 pandemic. [Cited 2023 October 16]. Available from: https://www.who.int/data/stories/global-excess-deaths-associated-with-covid-19-january-2020-december-2021
3. Gambichler T, Boms S, Susok L, Dickel H, Finis C, Abu Rached N, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol 2022;36:172-180
Google Scholar
4. Nakashima C, Kato M, Otsuka A. Cutaneous manifest- ations of COVID-19 and COVID-19 vaccination. J Dermatol 2023;50:280-289
Google Scholar
5. Bellinato F, Maurelli M, Gisondi P, Girolomoni G. Cuta- neous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med 2021;10:5344
Google Scholar
6. Pourani MR, Shahidi Dadras M, Salari M, Diab R, Namazi N, Abdollahimajd F. Cutaneous adverse events related to COVID-19 vaccines: A cross-sectional questionnaire-based study of 867 patients. Dermatol Ther 2022;35: e15223
Google Scholar
7. Català A, Muñoz-Santos C, Galván-Casas C, Roncero Riesco M, Revilla Nebreda D, Solà-Truyols A, et al. Cuta- neous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases*. Br J Dermatol 2022;186:142-152
Google Scholar
8. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 2020;34: e212-e213
Google Scholar
9. Hatami P, Asl HN, Aryanian Z. Cutaneous manifestations of COVID-19 in children: Practical points for clinicians. J Skin Stem Cell 2021;8:e122260
Google Scholar
10. World Health Organization (WHO)-Uppsala Monitoring Centre. The use of the WHO-UMC system for stand- ardized case causality assessment. Available from: http:// www.who-umc.org/Graphics/24734.pdf
11. Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immuno- genicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396:1979-1993
Google Scholar
12. Bae S, Lee YW, Lim SY, Lee JH, Lin JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci 2021;36:e115
Google Scholar
13. Robinson LB, Fu X, Hashimoto D, Wickner P, Shenoy ES, Landman AB, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. JAMA Dermatol 2021;157:1000-1002
Google Scholar
14. Alemayehu A, Demissie A, Yusuf M, Abdullahi Y, Abdulwehab R, Oljira L, et al. COVID-19 vaccine side effect: age and gender disparity in adverse effects fol- lowing the first dose of AstraZeneca COVID-19 vaccine among the vaccinated population in Eastern Ethiopia: a community-based study. SAGE Open Med 2022;10: 20503121221108616
Google Scholar
15. Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. First month of COVID-19 vaccine safety monitoring - United States. MMWR Morb Mortal Wkly Rep 2021;70: 283-288
Google Scholar
16. Urakawa R, Isomura ET, Matsunaga K, Kubota K, Ike M. Impact of age, sex and medical history on adverse re- actions to the first and second dose of BNT162b2 mRNA COVID-19 vaccine in Japan: a cross-sectional study. BMC Infect Dis 2022;22:179
Google Scholar
17. Siegrist CA. Mechanisms underlying adverse reactions to vaccines. J Comp Pathol 2007;137:S46-S50
Google Scholar
18. Bostan E, Gulseren D, Gokoz O. New-onset leukocyto- clastic vasculitis after COVID-19 vaccine. Int J Dermatol 2021;60:1305-1306
Google Scholar
19. Mücke VT, Knop V, Mücke MM, Ochsendorf F, Zeuzem S. First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: a case report. BMC Infect Dis 2021;21:958
Google Scholar
20. Cyrenne BM, Al-Mohammedi F, DeKoven JG, Alhusayen R. Pityriasis rosea-like eruptions following vaccination with BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol 2021;35:e546-e548
Google Scholar
21. für das Alter NA. Risikofaktor Komorbi-ditäten bei COVID-19-Erkrankung. Pneumologie 2020;74:639-640
22. Tan CC, Dofitas BL, Frez MLF, Yap CDD, Uy JKK, Ciriaco-Tan CP. Cutaneous manifestations of COVID-19 in a tertiary COVID-19 referral hospital in the Philippines. JAAD Int 2022;7:44-51
Google Scholar
23. Galván Casas C, Catalá A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, et al. Classification of the cutaneous manifest- ations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020;183:71-77
Google Scholar
24. de Masson A, Bouaziz JD, Sulimovic L, Cassius C, Jachiet M, Ionescu M, et al. Chilblains is a common cutaneous finding during the COVID-19 pandemic: A retrospective nationwide study from France. J Am Acad Dermatol 2020;83:667-670
Google Scholar
25. Gottlieb M, Long B. Dermatologic manifestations and complications of COVID-19. Am J Emerg Med 2020;38: 1715-1721
Google Scholar
26. Tamai M, Sakamoto R, Goto N, Morimura O, Nishida T, Iwahashi H, et al. Cutaneous manifestations of corona- virus disease 2019 patients in Japan. J Dermatol 2022; 49:872-878
Google Scholar
Congratulatory MessageClick here!