pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Lazara Alacán Pérez,Julio César Fernández Travieso,Evelyn Anie González Pla,Yeni González Liriano,Olaine Regla Gray Lovio,Elizabeth Sanler Wong,Maylen Rodríguez Ordaz,Yanela Rodríguez Lorenzo,Marlenys Pérez Bruzón,Jose Alejandro Díaz Cardoso,Ada Bermudez Sori,Juana del Valle Rodríguez,Elvia Nelemi Santos González,Lisandra Linares Luna,Ana Labrada Carballo,Yenney Reyes Nuñez,Yanay Fernández Domínguez,Manuel Eduardo Cordero Hernández,Sarahí Mendoza Castaño,Gladys Jiménez Rivero,Marta Lidia Barrera Moreira,Rolando Uranga Piña
10.17966/JMI.2024.29.3.98 Epub 2024 October 11
Abstract
Background: Dermatophytosis are the leading cause of fungal infections worldwide.
Objective: To compare the efficacy and safety of ozonated sunflower oil versus ketoconazole and terbinafine in patients with dermatophytosis.
Methods: Multicenter Phase III study, it had an open, randomized, comparative design, with three parallel group to which ozonated sunflower oil was apply, or ketoconazole, or terbinafine, twice a day for 6 weeks. The effect on the clinical and mycological cure rate was prefix as the primary efficacy variable. The effect on treatment time to achieve cure was consider as a secondary efficacy variable. All data were analyzed by the intention-to-treat method.
Results: The study included 300 patients of both sexes with a diagnosis of dermatophytosis, who were randomly distributed into three groups that received ozonated sunflower oil, or ketoconazole, or terbinafine, respectively. Treatment with ozonated sunflower oil produced a total clinical and mycological cure rate in 78% of patients, similar to ketoconazole (78%) and terbinafine (77%) treatment. Complete mycological cure was achieved in 87% of patients treated with ozonated sunflower oil, similar to ketoconazole (88%) and terbinafine (89%) treatments. Regarding the healing time, of the 233 patients with complete clinical cure, 10 patients (4.3%) were completely cure after 2 weeks of treatment, 58 patients (24.9%) after 4 weeks and 164 patients (70.4%) after 6 weeks. The treatments were safe and well tolerated.
Conclusions: It's concluded that ozonated sunflower oil applied for six weeks showed comparable efficacy and safety with ketoconazole and terbinafine in patients with dermatophytosis.
Keywords
Dermatophytes Ketoconazole Ozonated sunflower oil Terbinafine Tinea pedis
Dermatophytosis of the feet or tinea pedis is a fungal infection of the superficial layers of the skin produced by dermatophytes. Dermatophytes are divided into the following genera: Microsporum, Trichophyton, and Epidermophyton1-3. These mycoses constitute a serious public health problem worldwide, impacting millions of individuals annually and accounting for 28.5% of dermatology consultations in Cuba4,5. Dermatophytosis can be treated topically or systemically. Topical treatment uses several drugs in the form of creams, lotions, or ointments, including ketoconazole and terbinafine6-10.
Ozonated sunflower oil, a clear viscous liquid with a characteristic odor and milky appearance, has been approved for the treatment of dermatophytosis in Cuba. This liquid is a complex mixture consisting mainly of unsaturated triglycerides, modified triglycerides with acid functions, aldehydes, peroxides, and water in emulsion11,12. Experimental studies on ozonated sunflower oil have demonstrated its antifungal and antibacterial activities, whereas experimental toxicology studies have demonstrated its safety, with no toxicity having been associated with its use13-23. Clinical studies have found that ozonated sunflower oil was an effective, safe, and well-tolerated treatment for various dermatological diseases24-32.
Considering the aforementioned background, one can logically assume that ozonated sunflower oil could produce benefits similar to those of ketoconazole and/or terbinafine in patients with dermatophytosis. As such, the current study aimed to compare the efficacy and safety of ozonated sunflower oil with those of ketoconazole and terbinafine in the treatment of dermatophytosis, as well as evaluate its safety and tolerability.
This study was conducted in accordance with the principles reflected in the Helsinki Declaration, as well as the recommendations of the World Health Organization and the Cuban regulations on Good Clinical Practices. Our study protocol was approved by the Ministry of Public Health and by the Ethics Committee in Clinical Research of the ¨Manuel Fajardo¨ (IRB 9-2019) and ¨Carlos Juan Finlay¨ (IRB 2-2020) Hospitals, and the Cuban State Drug Control Centre. This study was registered in the Cuban Public Registry of Clinical Trials (RPCEC-00000289).
1. Study design
This phase III study employed a multicenter, open-label, randomized design to compare the efficacy of ozonated sunflower oil with that of ketoconazole and terbinafine applied twice daily for 6 weeks. The study consisted of the following five visits: a recruitment visit in which the initial history was taken and the tests to be performed were indicated (visit 1); an inclusion visit in which the treatments were delivered to the patients who satisfied the selection criteria (visit 2); and three follow-up visits at 2, 4 and 6 weeks of treatment (visits 3, 4, and 5, respectively). During each visit, a physical examination was performed, with monitoring of adherence to treatment and recording of adverse events being conducted in visits 3~5. Clinical and microbiological laboratory tests were conducted prior to visit 2 and at the end of treatment.
2. Diagnostic criteria (recruitment)
Patients with dermatophytosis who attended the Derma- tology Consultations of Manuel Fajardo and Carlos Juan Finlay Hospitals were recruited. A diagnosis of dermatophytosis of the lower limbs was established based on clinical criteria and microbiological confirmation. The clinical criteria included skin lesions characteristic of these conditions, such as scales, macerated areas, vesicles, and erythema, whereas micro- biological confirmation was performed through direct micro- scopic observation of the clinical material and culture of the sample in an appropriate mycological medium. These microbiological studies demonstrated the presence of dermatophytes.
3. Inclusion criteria
Males or females ≥18 years of age who had a positive clinical and microbiological diagnosis of dermatophytosis were included. We also included patients who had no other superimposed infection, were treatment-naïve, or did not receive specific topical or systemic treatment for more than 5 days after having signed their informed consent.
4. Exclusion criteria
Patients with diagnosed neoplasms, severe septic states, history of liver failure, history of kidney failure, decompensated chronic diseases, alcoholism, pregnancy, concomitant use of corticosteroids, cytostatics or immune-suppressants and antibiotics, and usual clinical history of drug allergies were excluded.
5. Treatment interruption criteria
Treatment was interrupted when no clinical improvement was observed, the patient did not desire to continue for any reason, serious adverse events occurred, and major violations of the study protocol were noted (lack of adherence to the treatment application for > 3 days and/or use of systemic antifungals other than those indicated).
6. Treatments groups
Group 1 consisted of those who received ozonated sunflower oil emulsion; Group 2 consisted of those who received ketoconazole 2% cream; and Group 3 consisted of those who received terbinafine 1% cream. All dermatophytosis treatments were topical, which would facilitate the evaluation of their effects. The ozonated sunflower oil emulsion or creams were applied on the affected area.
This study was conducted in outpatient settings, and treatments were applied to the affected areas twice a day (in the morning and evening after bathing) for 6 weeks. The doses of ozonated sunflower oil, ketoconazole, and terbinafine administered in this study were the same as those generally recommended in clinical practice throughout our country for the management of these patients. Moreover, the proposed treatment duration of 6 weeks corresponded with a period in which the effects of these treatments on dermatophytosis could be observed. The use of specific medications, such as superficial radiotherapy, mud therapy, or systemic antifungals, other than the study treatments was prohibited.
7. Primary efficacy endpoints
1) Clinical and mycological cure rate
Clinical cure was defined as the absence of skin lesions on the feet (soles, edges and interdigital spaces) characteristic of these conditions. Mycological cure was defined as negative direct microbiological examination and culture. Clinical and mycological cure was achieved when both conditions were satisfied.
Treatments were considered effective when clinical and mycological cure was noted after 6 weeks of treatment. Treatments were considered equivalent when patient responses were similar. A total response was considered when the treatments promoted clinical and mycological cure. A partial response was considered when the treatments promoted clinical improvement of the lesions.
8. Secondary efficacy endpoints
As secondary efficacy endpoints, we determined the duration of treatment needed to achieve cure.
9. Safety and tolerability
Data obtained from a physical examination (bodyweight, pulse rate, and arterial pressure), laboratory indicators and requests for adverse events (AEs) were included for safety and tolerability analysis. All undesirable events that newly appeared in the patients during the trials, regardless of cause, were considered AEs and classified as mild, moderate, or severe according to their intensity33.
10. Laboratory analyses
For laboratory analysis, venous blood samples were obtained under fasted conditions of no less than 12 h or no more than 16 h. Hematological variables (hemoglobin, hematocrit, red blood cell count, and white blood cell count) were deter- mined automatically using a Hematological Complex. Blood biochemistry variables (aspartate amine transferase, alanine amine transferase, glucose, and creatinine) were determine by enzymatic methods using reagent kits (Roche, Switzerland). Determinations were made using automated equipment available in the clinical laboratories of both hospitals.
For microbiological examination, tinea pedis lesions were disinfected with 70% ethanol, after which samples were collected a sterile Petri dish by scraping the periphery of the lesion using a sterile scalpel. For direct microscopic examination, a drop of 10% KOH was placed on the slide and mixed with a small portion of the material to be examined (skin sample). The slide was then gently placed over a low flame of a Bunsen burner to facilitate clearing and observe septate hyphae and arthrospores.
Samples positive to direct examination were seeded on Sabouraud Agar supplemented with chloramphenicol using an inoculation loop, incubated at room temperature for 30 days, and examined for growth every 5 days, one replicate of which was incubated. Plates with growth were micro- cultured. A sterilized glass Petri dish was used for the micro- culture, the bottom of which was covered with filter paper on which a "V" shaped glass rod, a coverslip, and a slide were placed. A portion of supplemented Papa Dextrose Agar with a surface of 1 cm2 was placed on the latter, where small fragments of the isolates to be identified were inoculated on the surface of the lateral edges. The culture medium was then covered with a sterile coverslip and incubated in a humid chamber at 37℃. Once fungal growth was detected, the coverslip was removed and placed on a slide containing a drop of lactophenol cotton blue. The preparation was observed under a microscope at 100× and × magnification.
11. Statistical analysis
Data analysis was performed according to the intention-to-treat method, including all randomized patients regardless of compliance with the treatments studied, and data imputation using the carryover method. Variables with categorical values were presented in contingency tables with absolute values, proportions, and percentages for each category using the Pearson χ2 test.
To compare the results between the three study groups, quantitative variables were analyzed using a nonparametric analysis of variance, such as the Kruskal-Wallis, and comparisons were made in pairs, always evaluating the homogeneity of the variances. For the "before versus after" tests of two means, the McNemar test was used, assuming a normal dis- tribution, and the nonsignificant signed-rank test. All statistical tests were two-tailed, and the level of significance established a priori for all the statistical tests was α = 0.05.
Data management and statistical analysis were performed in the Department of Data Management and Processing of National Clinical Trials Coordinators Centre, Havana, Cuba.
1. Baseline characteristics and group homogeneit
A total of 332 patients were recruited, among whom 300 were included in the active treatment phase. The reasons for noninclusion included negative mycological examination (9 patients), travel abroad (1 patient), and failure to perform the indicated examinations and analyses (22 patients). Table 1 summarizes the main characteristics of the study population, which were consistent with the selection criteria and statistically similar in all groups. This indicated that all groups were homogeneous, making randomization optimal.
|
|
Ketoconazole |
Terbinafine |
Ozonated sunflower oil |
Total |
|
Age (years) (X ± SD) |
52±16.1 |
51±14.9 |
50±15.3 |
51±15.4 |
|
Body mass index (kg/m2) (X ± SD) |
26.3±4.2 |
26.3±4.6 |
26.5±4.1 |
26.4±4.3 |
|
Sex (%) |
|
|
|
|
|
Male |
30.5 |
36.0 |
33.5 |
67.7 |
|
Female |
39.2 |
27.8 |
33.0 |
32.3 |
|
Personal
history (%) |
|
|
|
|
|
Overweight |
44.0 |
35.0 |
40.0 |
39.7 |
|
Arterial
hypertension |
40.0 |
40.0 |
34.0 |
38.0 |
|
Obesity |
18.0 |
27.0 |
22.0 |
22.3 |
|
Diabetes
mellitus |
10.0 |
9.0 |
10.0 |
9.7 |
|
Smoking |
12.0 |
6.0 |
10.0 |
9.3 |
|
Coronary
disease |
4.0 |
4.0 |
2.0 |
3.3 |
|
Dyslipidemia |
0.0 |
1.0 |
1.0 |
0.7 |
|
Concomitant
medications (CM) (%) |
|
|
|
|
|
Patients consuming CM |
49.0 |
50.0 |
41.0 |
46.7 |
|
ACEI |
19.0 |
24.0 |
19.0 |
20.7 |
|
Diuretics |
24.0 |
14.0 |
17.0 |
18.3 |
|
Calcium antagonists |
18.0 |
11.0 |
9.0 |
12.7 |
|
Antidiabetics |
9.0 |
4.0 |
8.0 |
7.0 |
|
Analgesics |
2.0 |
6.0 |
5.0 |
4.3 |
|
β-blockers |
7.0 |
1.0 |
3.0 |
3.7 |
|
Bronchodilators |
3.0 |
4.0 |
1.0 |
2.7 |
|
Antiplatelets |
2.0 |
1.0 |
1.0 |
1.3 |
|
Vasodilators |
0.0 |
1.0 |
1.0 |
0.7 |
|
Antigouty |
1.0 |
0.0 |
1.0 |
0.7 |
|
X: mean, SD: standard deviation, n: number of
patients, ACEI: angiotensin-converting enzyme inhibitors The table includes only those CM consumed by ≥ 2 patients. All comparison were not
significant (ANOVA, χ2 test). |
||||
The included patients had a history of other pathological conditions aside from dermatophytosis, such as overweight (39.7%), high blood pressure (38%), obesity (22.3%), dia- betes (9.7%), smoking (9.3%) and coronary heart disease (3.3%). The frequency of concomitant therapy was 46.7%, which was similar in all groups and consistent with their clinical history, highlighting the consumption of antihypertensives (angiotensin converting enzyme inhibitors, diuretics, calcium antagonists, and β-blockers), followed by oral hypo- glycemic agents, analgesics, bronchodilators, supplements, antiplatelets, vasodilators, and antigout medications.
2. Analysis of attrition
A total of 11 patients (3.7%) withdrew from this study. The causes of treatment interruption were adverse events (1 in the ketoconazole-treated group, 1 in the terbinafine-treated group, and 3 in the ozonated sunflower oil-treated group), voluntary abandonment (1 in the ozonated sun- flower oil-treated group), no clinical improvement (1 in the ketoconazole-treated group, 1 in the terbinafine-treated group, and 1 in the ozonated sunflower oil-treated group), and protocol violation (1 in the terbinafine-treated group and 1 in the ozonated sunflower oil-treated group).
With the exception of the 11 patients who were discharged, the rest of the included patients correctly applied the indicated treatments according to our evaluation of the empty bottles and tubes, as well as patient surveys, which showed excellent treatment adherence (greater than 90%) that was similar in all groups.
3. Analysis of efficacy
After 6 weeks, treatment with ozonated sunflower oil produced a total clinical and mycological cure rate of 78%, which was similar to the efficacy achieve in the groups treated with ketoconazole (78%) and terbinafine (77%). Another 16% of the patients treated with ozonated sunflower oil achieve partial clinical cure (improvement of the lesions) similar to that achieve in the groups treated with ketoconazole and terbinafine (15%) (Table 2 and Fig. 1).
|
Cure rate at the end of treatment (Week 6) |
Ketoconazole |
|
Terbinafine |
|
Ozonated sunflower oil |
|
% |
% |
% |
|||
|
Total
Clinical Healing (absence of injuries) |
78.0 |
|
77.0 |
|
78.0 |
|
Partial
Clinical Cure (presence of improved lesions) |
16.0 |
|
15.0 |
|
15.0 |
|
No
Clinical Cure (presence of lesions) |
6.0 |
|
8.0 |
|
7.0 |
|
Total
Mycological Healing (negative
direct examination and negative culture) |
88.0 |
|
89.0 |
|
87.0 |
|
Partial
Mycological Cure (direct
examination positive and culture negative) |
3.0 |
|
0.0 |
|
2.0 |
|
No
Mycological Cure (positive
direct examination and positive culture) |
9.0 |
|
11.0 |
|
11.0 |
|
Clinical
and Mycological Healing (absence
of lesions and negative culture) |
77.0 |
|
77.0 |
|
78.0 |
|
n:
number of patients The
comparisons between groups were not significant (χ2 test). |
|||||
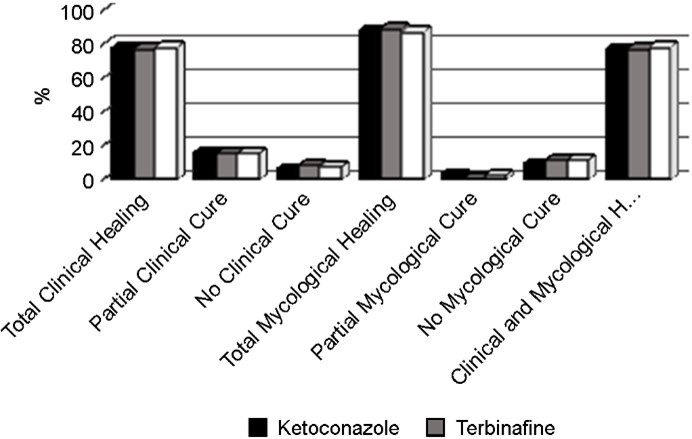
Only seven, six, and eight patients treated with ozonated sunflower oil, ketoconazole, and terbinafine did not achieve clinical cure, respectively. Complete mycological cure was achieved in 87% of the patients treated with ozonated sun- flower oil, whereas complete elimination of dermatophytes was achieved in 88% and 89% of those treated with keto- conazole and terbinafine, respectively (Table 2 and Fig. 1).
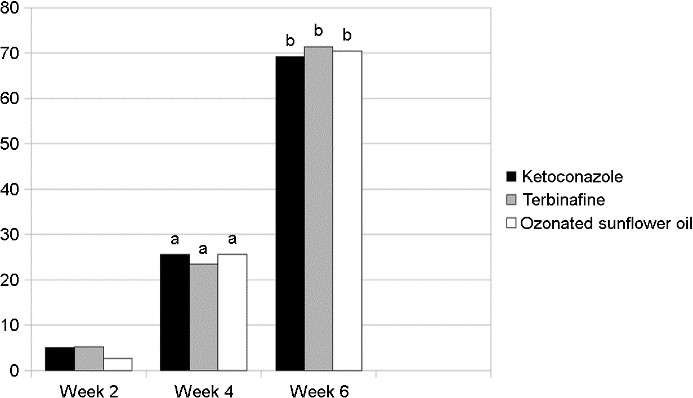
Regarding the healing time, among the 233 patients who achieved complete clinical cure, 10 (4.3%), 58 (24.9%), and 164 (70.4%) were completely cured after 2, 4, and 6 weeks of treatment, respectively, which was similar in all three groups (Table 3, Fig. 2).
|
Total clinical |
Ketoconazole (n = 78) |
|
Terbinafine (n = 77) |
|
Ozonated sunflower oil (n = 78) |
|||
|
n |
% |
n |
% |
n |
% |
|||
|
Week 2 |
4 |
5.1 |
|
4 |
5.2 |
|
2 |
2.6 |
|
Week 4 |
20a |
25.6 |
|
18a |
23.4 |
|
20a |
25.6 |
|
Week 6 |
54b |
69.2 |
|
55b |
71.4 |
|
55b |
70.5 |
|
n:
number of patients ap<0.01 Comparison versus week 2; bp<0.001 Comparison versus week 4 (McNemar test) All comparisons
between groups were not significant (χ2 test). |
||||||||
4. Analysis of safety and tolerability
After analyzing the effects of treatment on the physical and laboratory indicators investigated, we found no significant changes in any of the comparisons made (data not shown for simplicity).
Seven patients reported AEs during the study, among whom two reported worsening of the lesions, two developed lymphangitis, two developed superimposed sepsis, and one developed pruritus, all of which were classified as moderate given that they required treatment (two from the group treated with ketoconazole, one from the group treated with terbinafine, and four from the group treated with ozonated sunflower oil). However, all reported AEs had a controversial relationship with the treatments used.
The present study demonstrated that ozonated sunflower oil applied for 6 weeks improved the clinical and mycological cure rate (primary efficacy variable) in patients with dermatophytosis, with an efficacy, safety and tolerability comparable to those for ketoconazole and terbinafine. This study included 300 patients with a clinical and mycological diagnosis of dermatophytosis. The average age of the study population was 51 years, and the sex distribution showed male pre- dominance (203/300, 67.7%), which coincides with the findings reported internationally regarding its epidemiology34.
In addition, the distribution of other antecedents was also consistent with that described in other studies35, and the frequency of concomitant therapy was similar in all groups, which corresponded with their clinical history.
The baseline conditions of the treatment groups were homogeneous as demonstrated by the similarity of their demographic characteristics and main response variables, which indicates that the results obtained were attributed to the treatments investigated and not to the disparity in the initial condition of the groups compared.
Among the 300 included, 289 (96.3%) completed the study, a very satisfactory figure considering the substantial variability in dropout frequencies among studies in such patients. Furthermore, all patients who completed the study properly applied their medication, reinforcing the validity of the efficacy and safety data obtained36.
Treatment with ozonated sunflower oil prompted total clinical and mycological cure (primary efficacy variable) in 78% of the patients, which is considered satisfactory. Mean- while, another 16% of the patients treated with ozonated sunflower oil achieved partial clinical cure (improvement of the lesions), whereas 87% achieved complete mycological cure. However, comparisons performed at the end of treat- ment showed no significant differences between the groups. Therefore, all groups showed comparable efficacy according to the frequency of cases that achieved a clinically relevant response. All three groups showed a similar treatment duration to achieve cure (secondary efficacy variable).
Trichophyton mentagrophytes was the dermatophyte most frequently identified as the causal agent in the patients analyzed, followed by Epidermophyton floccosum and Tricho- phyton rubrum. These findings coincide with the results reported in other studies, which show that T. mentagrophytes continues to be the fungal agent causing the majority of tinea pedis cases37,38. After 6 weeks of treatment, a significant reduction was observed in the presence of the dermatophytes studied. However, no significant differences in the variables evaluated were noted between the groups at the end of treatment.
The current clinical study found that the application of ozonated sunflower oil for 6 weeks showed comparable efficacy to ketoconazole and terbinafine, which coincide with the findings reported by other authors for these pro- ducts in patients with epidermophytosis39-42. Moreover, our study found that the treatments showed a safety and tolerability profile consistent with data reported for these treatments7-10,25-32,39-42.
None of the treatments promoted changes in the physical examination or laboratory indicators investigated. Moreover, comparisons between the groups showed no significant differences, with the values remaining within the ranges considered normal for these variables.
The tolerability of ozonated sunflower oil was good considering that only four patients reported AEs, whereas two patients treated with ketoconazole and one with terbinafine also report AEs. All AEs reported during the study were classified as moderate because they required treatment and had a controversial relationship with the treatments used.
The current study showed that ozonated sunflower oil applied for 6 weeks showed comparable efficacy and safety to ketoconazole and terbinafine in patients with demartophytosis.
References
1. Jartarkar SR, Patil A, Goldust Y, Cockerell CJ, Schwartz RA, Grabbe S, et al. Pathogenesis, Immunology and management of dermatophytosis. J Fungi 2021;8:39
Google Scholar
2. Martinez-Rossi NM, Peres NTA, Rossi A. Pathogenesis of dermatophytosis: Sensing the host tissue. Mycopathologia 2017;182:215-227
Google Scholar
3. Segal E, Elad D. Human and zoonotic dermatophytoses: epidemiological aspects. Front Microbiol 2021;12:713532
Google Scholar
4. Martinez-Rossi NM, Peres NTA, Bitencourt TA, Martins MP, Rossi A. State-of-the-Art dermatophyte infections: epidemiology aspects, pathophysiology, and resistance mechanisms. J Fungi 2021;7:629
Google Scholar
5. Kovitwanichkanont T, Chong AH. Superficial fungal in- fections. Aust J Gen Pract 2019;48:706-711
Google Scholar
6. Moskaluk AE, VandeWoude S. Current topics in dermato- phyte classification and clinical diagnosis. Pathogens 2022; 11:957
Google Scholar
7. Durdu M, Ilkit M, Tamadon Y, Tolooe A, Rafati H, Seyedmousavi S. Topical and systemic antifungals in der- matology practice. Expert Rev Clin Pharmacol 2017;10: 225-237
Google Scholar
8. Lopes AI, Tavaria FK, Pintado ME. Conventional and natural compounds for the treatment of dermatophytosis. Med Mycol 2020;58:707-720
Google Scholar
9. Gaurav V, Bhattacharya SN, Sharma N, Datt S, Kumar P, Rai G, et al. Terbinafine resistance in dermatophytes: Time to revisit alternate antifungal therapy. J Mycol Med 2021;31:101087
Google Scholar
10. Ward H, Parkes N, Smith C, Kluzek S, Pearson R. Con- sensus for the treatment of tinea pedis: A systematic review of randomised controlled trials. J Fungi 2022;8: 351
Google Scholar
11. Ugazio E, Tullio V, Binello A, Tagliapietra S, Dosio F. Ozonated oils as antimicrobial systems in topical appli- cations. Their characterization, current applications, and advances in improved delivery techniques. Molecules 2020;25:334
Google Scholar
12. Molerio J, Menéndez S, Díaz W, Lezcano I, León F, Ledea O, et al. Health record on the application of ozonated sunflower oil in Epidermophytosis, #1498, CECMED 1999
13. Díaz MF, Gavín JA, Gómez M, Curtielles V, Hernández F. Study of ozonated sunflower oil using 1H NMR and microbiological analysis. Ozone Sci Eng 2006;28:59-63
Google Scholar
14. Ledea Lozano O, Curtiellas V, Moleiro J, Garcés R. Evi- dence of the oxidizing mechanism in the antibacterial activity of ozonated sunflower oil. Rev CENIC Chemical Sciences, Special Issue, 2010:41
15. Hormigo MA. Therapeutic efficacy of ozonated sunflower oil against Malassezia pachydermatis infection in dogs and cats. Rev Esp Ozone Therapy 2015;5:55-74
16. Lezcano I, Nuñez N, Espino M, Gómez M. Antibacterial activity of ozonized sunflower oil, Oleozón, against Staphylococcus aureus and Staphylococcus epidermidis. Ozone Sci Eng 2000;22:207-214
Google Scholar
17. Lezcano I, Moleiro J, Gómez M, Contreras R. In vitro activity of ozonated oil against etiological agents of skin infections. Rev CENIC Biological Sciences 1998;29:209-212
18. Rodríguez MD, Menéndez S, Gómez M. Teratogenic study of ozonated sunflower oil. First Ibero-Latin American Congress of Ozone Applications 1990. CNIC-CIMEQ.
19. Arteaga P, Mirabal JM, Bada AM, González B, Zamora Z, Remigio AC. Toxicological classification of ozonated oil. Rev. CENIC Biological Sciences 2001;32:57-58
20. Díaz M, García G, García K, Sánchez Y, Tillan J. Evalu- ation of ophthalmic, dermal irritability and the sensitizing effect of Oleozon® topic. Rev Elect Veterinaria REDVET 2006;7:1-6
Google Scholar
21. Acevedo A, González F, Molerio J, González B, León A, Hernández J. 120-day subchronic dermal toxicity test of ozonated oil in Cenp rats SPRD. Ad Biotech Mod 1997; 4:1-4
22. Sánchez GM, León SF, Rodriguez TC, Merino NC, Sam SR, Cedeño MP, et al. Study of the acute dermal toxicity of ozonated oil in rats. Rev CENIC Biological Sciences 1997; 28:35-38
23. Remigio AC, González Y, Zamora Z, Fonseca G, Molerio J. Genotoxic evaluation of ozonated oil using micronucleus assays in mouse bone marrow and peripheral blood. Rev CENIC Biological Science 1998;29:200-202
24. Menéndez S, González R, Ledea E, Hernández F, León OS, Díaz M. Ozono. Basic aspects and clinical applications. Havana, Cuba: CENIC Editorial 2008a;10-320
25. Lu J, Fu Z, Liu S, Huang J, Li J, Huang J, et al. Safety evaluation for medical ozone oil on skin. J Cent South Univ Med Sci 2018;43:131-138
Google Scholar
26. Menéndez S, Falcón L, Simón RD, Landa N. Efficacy of ozonated sunflower oil in the treatment of tinea pedis. Mycoses 2002;45:329-333
Google Scholar
27. Menéndez S, Re L, Falcón L, Argote MB, Méndez I, Fernández D, et al. Safety of Ozonaed sunflower oil in the treatment of tinea pedis: phase IV clinical trial. Int J Ozone Therapy 2008;7:55-59
Google Scholar
28. Menéndez S, Falcón L, Maqueira Y. Therapeutic efficacy of topical OLEOZON® in patients suffering from onycho- mycosis. Mycosis 2011;54:e272-277
Google Scholar
29. Menéndez S, Fernández M, Amoroto M, Uranga R, Acuña P, Elisa Benítez J, et al. Efficacy and safety of ozonated sunflower oil in the treatment of patients with impetigo. Rev Panam Infectol 2007;9:23-29
30. Lincheta LF, Cepero SM, Simón RD, Otano EG, Duque SM, Garcia MA. Ozonated oil in dermatology. 9 years experience. Rev CENIC Biological Sciences 1998;29:192-195
31. Argote M. Generalization of the use of ozonated sun- flower oil in the treatment of Epidermophytosis of the feet. Thesis in option to the degree of 1st Degree spe- cialist in Dermatology, H.M. Dr. Carlos J Finlay. Havana city 2006
32. Falcón L, Landa N, Moya S. Solution for Epidermophytosis of the feet in members of the FAR. Cuban Military Medicine Journal 2000;29:98-102
33. Requirements for the notification and reporting of serious and unexpected adverse events in clinical trials. Regula- tion No. 45-2007. Center for State Control of Medicines, Equipment and Medical Devices (CECMED), MINSAP, Havana, Cuba, 2007
34. Urban K, Chu S, Scheufele C, Giesey RL, Mehrmal S, Uppal P, et al. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: A cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int 2020;2:22-27
Google Scholar
35. Hayette MP, Sacheli R. Dermatophytosis, Trends in epi- demiology and diagnostic approach. Curr Fungal Infect Rep 2015;9:164-179
Google Scholar
36. Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol 2015;41:374-388
Google Scholar
37. Chollet A, Cattin V, Fratti M, Mignon B, Monod M. Which fungus originally was Trichophyton mentagrophytes? Historical review and illustration by a clinical case. Myco- pathologia 2015;180:1-5
Google Scholar
38. Gnat S, Nowakiewicz A, Zięba P. Taxonomy of dermatophytes-the classification systems may change but the identification problems remain the same. Postępy Mikrobiologii-Advancements of Microbiology 2019;58: 49-58
Google Scholar
39. Choi FD, Juhasz MLW, Atanaskova Mesinkovska N. Topical Ketoconazole: a systematic review of current dermatological applications and future developments. J Dermatolog Treat 2019;30:760-771
Google Scholar
40. Gupta AK, Foley KA, Versteeg SG. New antifungal agents and new formulations against dermatophytes. Myco- pathologia 2017;182:127-141
Google Scholar
41. Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: a comprehensive review. Indian Dermatol Online J 2016;7:77-86
Google Scholar
42. Maxfield L, Preuss CV, Bermudez R. Terbinafine. In: Stat- Pearls [Internet]. Treasure Island (FL): StatPearls Publishing 2023; Jan. 2023 May 29. PMID:3142480
Google Scholar
Congratulatory MessageClick here!