pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Kartika Misalina,Muhammad Yulianto Listiawan,Rahmadewi,Dwi Murtiastutik,Cita Rosita Sigit Prakoeswa,Afif Nurul Hidayati,Budi Utomo
10.17966/JMI.2024.29.2.70 Epub 2024 July 07
Abstract
Background: Erythema nodosum leprosum (ENL) is an acute immunologic complication of multibacillary leprosy (MB) that causes systemic inflammation in various organs. It is a major factor contributing to morbidity, mortality, and financial hardship. ENL is diagnosed clinically, and objective indicators for severe conditions remain unexplored. Simple blood biomarkers that differentiate between different ENL disease severity levels are required. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been widely studied as severity biomarkers of numerous neoplastic and inflammatory disorders.
Objective: We examined NLR and PLR values at various ENL severities and their role as severe disease indicators for ENL.
Methods: This cross-sectional retrospective study examined 246 patients with multibacillary leprosy—with and without ENL reactions and aged 18 and above—and calculated their NLR and PLR values. Then, we compared patients with mild and severe disease states to those without ENL. The severity classification was based on the ENLIST ENL Severity Score (EESS). To determine the NLR and PLR cutoff points of mild and severe ENL, a receiver operating characteristic (ROC) curve was constructed.
Results: The NLR value for severe ENL was significantly higher than that for mild ENL (p < 0.05), with a severe disease cutoff point of 5.71 (95.7% sensitivity, 68.7% specificity). No significant differences were found in PLR between patients with mild and severe ENL.
Conclusion: While PLR is not an effective assay for evaluating ENL severity, NLR is a potential biomarker for severe ENL reactions in patients with multibacillary leprosy.
Keywords
Erythema nodosum leprosum Neglected disease Neutrophil-to-lymphocyte ratio Platelet-to-lymphocyte ratio Severity
Erythema nodosum leprosum (ENL) is an immunologic reaction that develops as a multibacillary (MB) leprosy com- plication, affecting the skin, nerves, and other organs. A systematic review of field studies showed the incidence of ENL ranging from 1 to 8 per 100 person-years1. An Indonesian hospital study revealed that 33.4% of MB leprosy patients experienced an ENL reaction2 and that these patients were susceptible to nerve injuries, potentially leading to significant disability, morbidity, socio-economic burdens, and reduced quality of life1. ENL can manifest as mild (46.2%) to severe (5.38%) inflammatory conditions3. Clinical manifestations of severe inflammation may be invisible; however, cell-level changes are occurring by this time. Objective markers that predict subclinical inflammatory progression of ENL are needed to prevent prolonged treatment time and predict clinical worsening4.
Among the various simple blood test parameters, the neu- trophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are uniquely associated with systemic inflam- mation5. They are used as systemic inflammatory markers to predict prognosis and evaluate severity. These ratios are considered relatively stable and more accurate than absolute neutrophils (ANC), absolute lymphocytes (ALC), or platelet (PLC) counts6.
ENL can be characterized through neutrophil activities. A recent study found that peripheral neutrophil counts, mainly low-density (LDN), tend to be elevated, particularly in cases of severe ENL7. Platelets, or thrombocytes, actively participate in inflammatory responses by creating microthrombi that aid in trapping pathogens during the cellular immune response8. Patients with ENL often demonstrate increased T-helpers, reduced T-cytotoxic lymphocytes, and higher T-helper-to-T-lymphocyte ratios than controls; usually, these are the only lymphocytes involved in ENL.
Increases in NLR and PLR were previously associated with ENL incidence in patients with MB leprosy9. The NLR has a diagnostic accuracy of 78% for leprosy reactions10. Further, NLR and PLR have been widely studied as biomarkers for various systemic inflammatory diseases, including ulcerative colitis, rheumatoid arthritis, asthma, systemic lupus erythema- tosus, infection, and various malignancies.
NLR can predict clinical outcomes and severity in individuals with bacteremia or community-acquired pneumonia10,11. However, NLR and PLR have not been explored as biomarkers of ENL severity and prognosis. The identification of accurate biomarkers will improve our ability to evaluate disease progression and therapy outcomes. NLR and PLR are available and readily obtained within standard clinical practice settings. Therefore, this study investigated the use of NLR and PLR in different disease severities and assessed the significance of NLR and PLR as biomarkers for severe ENL.
1. Study design
This cross-sectional retrospective study was carried out at the Leprosy Division of Dr. Soetomo General Academic Hospital in Surabaya, Indonesia. The data was gathered from the medical records of individuals with multibacillary leprosy who visited the hospital between January 2017 and December 2021.
2. Patient selection
The study included patients 18 years and older who were diagnosed with multibacillary leprosy based on clinical and laboratory examination. Hematological data was obtained from the patient's complete blood count test on the day of diagnosis. Patients with HIV, autoimmune diseases, diabetes mellitus, other mycobacteriosis, Lucio's phenomenon, type 1 leprosy reaction, and other conditions were excluded. Patients administered steroids or other immunosuppressive medi- cations within the previous two weeks were also excluded.
3. Variables and definitions
The patient-related variables we collected included age, sex, body weight, height, leprosy type (according to Ridley-Jopling classification), bacterial index (BI), morphological index, onset of MDT therapy, ENL status, severity score (ENLIST ENL Severity Score [EESS]), and complete blood count results at the first outpatient visit.
Physicians experts in leprosy diagnosis and treatment diag- nosed all cases of ENL upon observing a sudden eruption of tender papules, nodules, or plaques plus three key symptoms (tender enlarged nerves, arthritis, lymphadenitis, increased loss of sensation or muscle power, epididymal-orchitis, edema of the face or extremities, and a positive Ryrie or Ellis test, and iridocyclitis or episcleritis). ENL severity was classified according to the EESS, which includes 10 variables worth 0~3 points and is used to classify ENL as mild (< =8 total points) or severe (> 8 total points).
Body weight and height were used to calculate each patient's body mass index (BMI) and assess their nutritional status. Skin smears from three distinct body sites were used to calculate all morphological and microbiological indexes. ANC, the ALC of peripheral blood, and the PLC and the ALC quotient were used to calculate the NLR and PLR, respectively.
4. Statistical analysis
For quantitative data that followed a normal distribution or did not, the mean (± standard deviation) or median (minimum–maximum), respectively, were used to tabulate the data. The Kolmogorov-Smirnov test was utilized to assess the normality of the distribution. The percentage of the total qualitative data value was calculated. The nonparametric Kruskal–Wallis test was employed to determine the association between the hematologic variable and the severity of ENL, as all examined parameters exhibited a nonnormal distribution. Spearman's rho correlation test was used to analyze the relationship between NLR and ENL severity and PLR and ENL severity. Furthermore, a receiver operating characteristic (ROC) curve was generated to assess the sensitivity and specificity of NLR and PLR as biomarkers for severe ENL.
1. Demographic study
There were 425 patients who visited the Leprosy Division between January 2017 and December 2021 (Table 1); 246 (average age 38.13 ± 14.47 years, 71.5% male, 28.5% female) patients with multibacillary leprosy met all inclusion and ex- clusion criteria. Of these, 39 (15.9%) had an ENL reaction, including 16 classified as mild (6.5%) and 23 as severe (9.3%). Most ENL patients, regardless of disease severity, were male (81.3% of mild patients and 60.9% of severe patients), aged 18~44 years (68.75% of mild and 78.3% of severe), and multidrug therapy-naive (62.5% of mild and 47.8% of severe). Mild (50.0%) and severe (56.5%) ENL was primarily observed in patients with lepromatous leprosy. Most patients with mild (62.5%) and severe (65.2%) ENL had a BI of 1~2 (62.5% of mild and 65.2% of severe) normal nutritional status, as indicated by a normal BMI (mild: 62.5%; severe: 52.2%). Mycobacterial indexes of 1~4 were primarily found in patients with mild ENL (50.0%).
|
Characteristics |
|
Non-ENL |
Mild
ENL |
Severe
ENL |
n
(%) |
|
Gender |
Male |
149 (72.0) |
13 (81.3) |
14 (60.9) |
176 (71.5) |
|
Female |
58 (28.0) |
3 (18.8) |
9 (39.1) |
70 (28.5) |
|
|
Age |
18~44 |
136 (65.7) |
11 (68.75) |
18 (78.3) |
165 (67.07) |
|
45~59 |
52 (25.1) |
3 (18.7) |
4 (15.4) |
59 (23.9) |
|
|
>60 |
19 (9.2) |
2 (12.5) |
1 (4.4) |
22 (8.94) |
|
|
BMI |
Underweight |
19 (9.2) |
1 (6.3) |
3 (13.0) |
23 (9.3) |
|
Normal |
119 (57.5) |
10 (62.5) |
12 (52.2) |
141 (57.3) |
|
|
Overweight |
36 (17.4) |
4 (25.0) |
6 (26.1) |
46 (18.7) |
|
|
Obese
(Grade I) |
28 (13.5) |
1 (6.3) |
1 (4.3) |
30 (12.2) |
|
|
Obese
(Grade II) |
5 (2.4) |
0 (0) |
1 (4.3) |
6 (2.4) |
|
|
Leprosy type |
BB |
114 (55.1) |
1 (6.3) |
0 (0) |
115 (46.7) |
|
BL |
54 (26.1) |
7 (43.8) |
10 (43.5) |
71 (28.9) |
|
|
LL |
39 (18.8) |
8 (50.0) |
13 (56.5) |
60 (24.4) |
|
|
BI |
0 |
98 (47.3) |
2 (12.5) |
0 (0) |
100 (40.7) |
|
1~2 |
56 (27.1) |
10 (62.5) |
15 (65.2) |
81 (32.9) |
|
|
³ 3 |
53 (25.6) |
4 (25.0) |
8 (34.8) |
65 (26.4) |
|
|
MI |
0 |
104 (50.2) |
7 (43.8) |
14 (60.9) |
125 (50.8) |
|
1~4 |
69 (33.3) |
8 (50.0) |
6 (26.1) |
83 (33.7) |
|
|
³ 5 |
34 (16.64) |
1 (6.3) |
3 (13) |
38 (15.4) |
|
|
Onset of MDT treatment |
New |
187 (90.3) |
10 (62.5) |
11 (47.8) |
208 (84.6) |
|
On
MDT therapy |
3 (1.4) |
1 (6.3) |
2 (8.7) |
6 (2.4) |
|
|
RFT |
0 (0) |
2 (12.5) |
5 (21.7) |
7 (2.8) |
|
|
Drop Out |
11 (5.3) |
2 (12.5) |
4 (17.4) |
17 (6.9) |
|
|
Relapse |
6 (2.9) |
1 (6.3) |
1 (4.3) |
8 (3.3) |
|
|
BI: Bacterial index; BMI: Body
mass index; MDT: Multidrug therapy; MI: Morphological index; RFT: Release
from treatment |
|||||
2. NLR and PLR analysis
The median ALC and ANC were substantially higher (p = 0.001) in ENL patients with severe disease than in mild or non-ENL controls (Table 2). Compared to mild and non-ENL group patients, severe ENL patients had significantly lower ANC (p = 0.001). No statistically significant between-group differences were found for PLC (p = 0.055).
|
Blood
count |
Non-ENL |
Mild
ENL |
Severe
ENL |
p value* |
|
Leukocytes |
7,580 |
11,190 |
17,500 |
0.001 |
|
Neutrophils |
4,980 |
8,675 |
16,130 |
0.001 |
|
Lymphocytes |
1,710 |
1,755 |
1,120 |
0.001 |
|
Platelet |
300,000 |
350,000 |
416,000 |
0.055 |
|
Values shown
median (minimum - maximum) *Non-parametric Kruskal-Wallis test |
||||
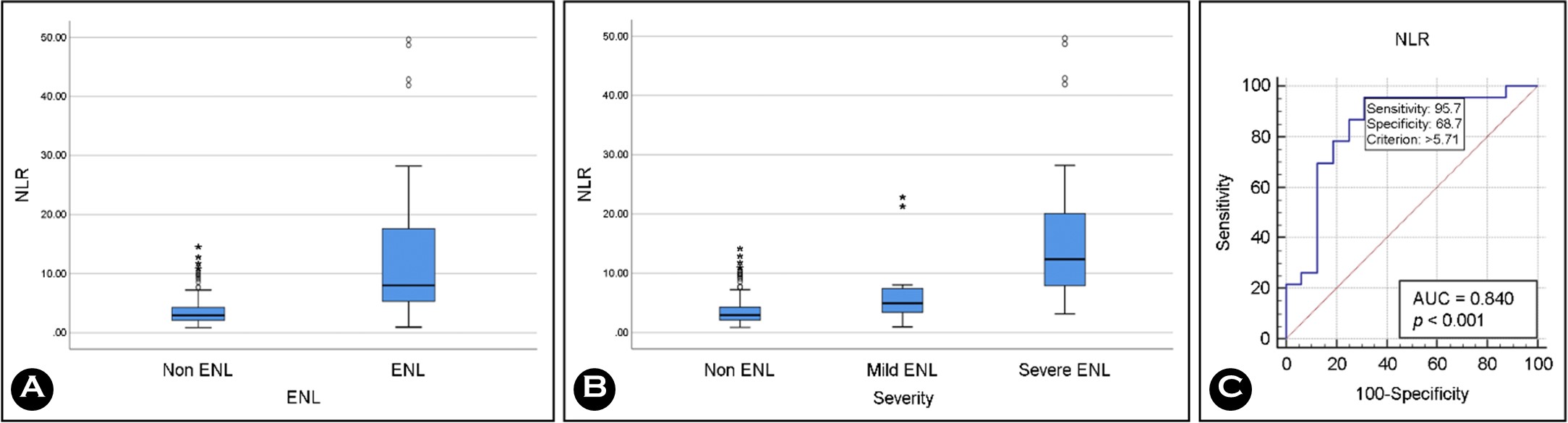
Table 3 shows a significant difference (p = 0.001) in median NLR between the non-ENL controls and ENL groups (mild and severe), which also reflected in Fig. 1A. Patients with severe ENL had a considerably higher median NLR (p = 0.001) than their mild ENL counterparts (Fig. 1B). Compared to patients in the ENL (mild and severe) groups, the median PLR of non-ENL patients was substantially lower. Nevertheless, there was no apparent distinction in the median PLR between patients with mild and severe ENL (p = 0.189).
|
|
Non ENL |
ENL |
Mild ENL |
Severe ENL |
P1(a) |
P2(a) |
Correlation |
|
NLR |
2.49 |
8.01 |
4.94 |
12.35 |
0.001 |
0.001 |
0.532; |
|
PLR |
169.89 |
259.38 |
226.08 |
292.17 |
0.001 |
0.189 |
0.300; |
|
Values shown
median (minimum - maximum) (a)Non-parametric Kruskal-Wallis test (b)Spearman's rho correlation test P1: P value between Non-ENL and ENL group P2: P value between mild and severe ENL
group ENL, erythema
nodosum leprosum. NLR, neutrophil-to-lymphocyte ratio. PLR,
platelet-to-lymphocyte ratio |
|||||||
ENL disease severity was positively correlated with PLR (r = 0.300) and NLR (r = 0.532). Among patients with severe ENL, the areas under the curve for PLR (0.625) and NLR (0.840) suggest that PLR is a poor indicator of severe disease but that NLR has modest utility. Fig. 1C shows the NLR cutoff value for severe ENL, which was 5.71 (sensitivity 95.7%, specificity 82.5%). The PLR cutoff value for severe ENL was 365.31 (sensitivity 39.1%, specificity 93.7%).
Inflammatory and immune responses, including innate and adaptive immunity, significantly influence ENL pathophysi- ology. Tumor necrosis factor (TNF)-α and other cytokines—including immune complexes, neutrophils, and different subtypes of lymphocytes such as B cells, T cells, helper T cells (Th)-17, and regulatory T cells (Treg) —are immuno- logical ENL response indicators12. NLR reflects the equilibrium between two facets of the immune response and may serve as a possible signal for identifying ENL. Tanojo and colleagues previously examined NLR and PLR in 182 consecutive patients with MB leprosy. Patients with an ENL reaction had a higher NLR (8.19 [2.9~21.46]) than patients without an ENL reaction (2.93 [0.86~12.85]) (p < 0.005)9. The present study's findings are essentially in line with those of Tanojo et al.'s investigation. Another similar study found increased NLR values in patients with leprosy reactions, especially those with type 2 or ENL10.
NLR reflects an increase in neutrophils and a reduction in lymphocytes. Across the board, lower NLR indicates conserved immunological balance and is typically associated with a favorable prognosis13. NLR increases in response to systemic inflammation10, and higher NLR is linked to increases in mor- tality14, exacerbations, dyspnea, and disease severity scores. It can even increase the risk of death from other inflammatory illnesses like COPD15.
The current study is the first to investigate whether the NLR can predict disease severity in ENL. Therefore, it is not possible to compare our results with previous ones. According to our current understanding of the immunological response in the ENL reaction, the study's findings demonstrate that severe cases are associated with high ANC and low ALC. Therefore, it seems reasonable that patients with severe disease would also present a high NLR. We confirmed this positive relationship between NLR and ENL severity, particu- larly in the patients with the most severe disease. In this study, the severe ENL group had a considerably higher median NLR (12.35 [3.13~49.64]) than the mild ENL group (4.94 [0.94~22.90]) (p < 0.005). Thus, NLR can determine ENL severity, with a cutoff value 5.71 (i.e., 95.7% sensitivity and 68.7% specificity) for severe disease.
Neutrophils are histological markers of ENL and release mediators that promote inflammation12. Increased NLR strongly correlates with systemic inflammation and indicates neutrophil-mediated, nonspecific acute inflammatory re- sponses. The superficial part of the neutrophil consists of cell surface receptors, namely CD64 (FcγRI). The cell surface receptor CD64 (FcγRI) expression is low in resting neutrophils; however, its expression increases when gram-negative bacteria stimulate the cells. Additionally, CD64 is an early biomarker and severity predictor for ENL. In situ and circulating neutro- phils in ENL express CD64, but this is not true in patients with nonreactional leprosy16. Most TNF-α and IL-8 linked to tissue damage in ENL are produced by neutrophils, which is in line with CD64's involvement in the elevation of proinflammatory cytokine production17.
Lymphocytes comprise various cell subtypes with unique roles in the adaptive immune response. Stress increases specific cell subtypes while decreasing others to maintain homeostasis. This agrees with our finding that there is no noticeable dis- tinction between mild ENL and non-ENL. A prior retrospective analysis shows that ALC alone cannot distinguish between ENL and non-ELN9. In patients with more severe inflammation, inflammatory process dysregulation can produce uncontrolled, persistent inflammation and additional tissue injury. We found patients with severe ENL had significantly lower ALC (1.120 [470~2.350]) than their mild counterparts (1.755 [680~ 2.950]) (p < 0.05). The low lymphocyte count in the severe ENL group was proportionate to the high NLR value observed in the same group, reflecting an immune imbalance that likely produced more severe inflammation18.
PLR is increasingly considered a novel systemic inflam- matory marker. Its application was first documented in neoplastic disease—including hepatocellular carcinoma and breast cancer—as a prognostic factor19. Platelets significantly impact immunomodulatory and inflammatory processes by triggering inflammatory cytokine release20 and interacting with various bacteria and immune cells, such as macrophages, T lymphocytes, neutrophils, and natural killer cells, to initiate or exacerbate inflammation21. Increased PLC is highly cor- related with increased systemic inflammation, potentially influencing the course and prognosis of numerous conditions, including diabetes mellitus and atherosclerosis20,21.
PLR can distinguish between MB leprosy patients with ENL reactions and those without ENL. The PLR value of the ENL patients (169.89 [61.14~766.33]) was significantly higher as compared to those without an ENL reaction (259.38 [59.66~ 1,172.34]) (p = 0.001). PLR may also serve as a diagnostic marker for the ENL reaction in patients with MB leprosy13. Nevertheless, we found that PLR did not correlate with ENL disease severity. The median PLR values were statistically similar in patients with mild (226.08 [59.66~670.59]) and severe (292.17 [87.76~1,172.34]) disease (p = 0.189).
The involvement of platelets in ENL pathogenesis needs to be better understood. A previous study found that patients with ENL had increased fibrinogen and platelet titers, pro- longed activated partial thromboplastin time (aPTT), and increased platelet activation. Increased PLC was seen in the early stages of the ENL reaction22. We found that the severity of the ENL reaction increased in conjunction with increases in PLC; however, this relationship was non-significant (p = 0.055). This result might support the insignificant difference in PLR value between mild and severe ENL mentioned before.
There are several limitations to our investigation. Based on administrative data, this retrospective, single-center study included a small number of patients in the case group. The complete blood count was evaluated in all MB leprosy patients who made their first visit to our outpatient clinic without distinguishing the chronicity of the ENL reaction. Disease biomarkers may be reflected differently in acute and chronic ENL. The inability to suppress the inflammatory response will progress from acute to persistent or chronic inflammation. Moreover, we did not assess the therapy outcome in the ENL patient. Therefore, further prospective investigations with follow-up periods are necessary to evaluate these parameters as treatment outcome predictors.
The NLR can help identify patients with severe inflammation as it closely correlates with ENL severity. This simple and easy method with relatively high sensitivity and specificity can support clinicians in detecting severe ENL in MB leprosy patients. PLR was not a useful biomarker of ENL severity. Future, well-powered, and prospective studies are necessary to confirm our findings.
References
1. Sales AM, Illarramendi X, Walker SL, Lockwood D, Sarno EN, Nery JA. The impact of erythema nodosum leprosum on health related quality of life in Rio de Janeiro. Lepr Rev 2017;88:499-509
Google Scholar
2. Fransisca C, Zulkarnain I, Ervianti E, Damayanti D, Sari M, Budiono B, et al. A retrospective study: epidemiology, onset, and duration of erythema nodosum leprosum in Surabaya, Indonesia. BIKKK 2021;33:8-12
Google Scholar
3. Baima de Melo C, Silva de Sa BD, Anibal Carvalho Costa F, Nunes Sarno E. Epidemiological profile and severity of erythema nodosum leprosum in Brazil: a cross-sectional study. Int J Dermatol 2020;59:856-861
Google Scholar
4. Luo Y, Kiriya M, Tanigawa K, Kawashima A, Nakamura Y, Ishii N, et al. Host-related laboratory parameters for leprosy reactions. Front Med 2021;8:694376
Google Scholar
5. Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I, Udovicic I, Andjelic T, et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia? Mediators Inflamm 2018;2018:e3758068
Google Scholar
6. Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018;97: e11138
Google Scholar
7. Tavares IF, Dos Santos JB, Pacheco FDS, Gandini M, Mariante RM, Rodrigues TF, et al. Mycobacterium leprae induces neutrophilic degranulation and low-density neu- trophil generation during erythema nodosum leprosum. Front Med 2021;8:711623
Google Scholar
8. Kumar KH, Chauhan A. Leprosy reactions: pathogenesis and clinical features. In: Kumar B, Kar HK, editors. IAL Textbook of leprosy. India: Jaypee Brothers Medical Publishers, 2017:416-437
9. Tanojo N, Damayanti, Utomo B, Ervianti E, Murtiastutik D, Prakoeswa CRS, et al. Diagnostic value of neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio in the diagnosis of erythema nodosum leprosum: a retrospective study. Trop Med Infect Dis 2022;7:39
Google Scholar
10. Gomes LT, Morato-Conceição YT, Gambati AVM, Maciel-Pereira CM, Fontes CJF. Diagnostic value of neutrophil-to-lymphocyte ratio in patients with leprosy reactions. Heliyon 2020;6:e03369
11. Lee H, Kim I, Kang BH, Um SJ. Prognostic value of serial neutrophil-to-lymphocyte ratio measurements in hospit- alized community-acquired pneumonia. Plos One 2021; 16:e0250067
Google Scholar
12. Indah MS, Karmila IG. Immunopathogenesis of erythema nodosum leprosum. Intisari Sains Medis 2021;12:969-973
Google Scholar
13. Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutro- phil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci 2022;23:3636
Google Scholar
14. Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep 2021;11:464
Google Scholar
15. Pascual-Gonzalez Y, Lopez-Sanchez M, Dorca J, Santos S. Defining the role of neutrophil-to-lymphocyte ratio in COPD: a systematic literature review. Int J Chron Obstruct Pulmon Dis 2018;5:3651-3662
Google Scholar
16. Schmitz V, Prata RB, Barbosa MG, Mendes MA, Brandão SS, Amadeu TP, et al. Expression of CD64 on circulating neutrophils favoring systemic inflammatory status in erythema nodosum leprosum. PLoS Neglected Tropical Diseases 2016;10:e0004955
Google Scholar
17. Schmitz V, Tavares IF, Pignataro P, Machado AM, Pacheco FDS, Dos Santos JB, et al. Neutrophils in leprosy. Front Immunol 2019;10:495
18. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018;9:7204
Google Scholar
19. Mertoglu C, Gunay M. Neutrophil-Lymphocyte ratio and platelet-lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr 2017;11:S127-S131
Google Scholar
20. Nording HM, Seizer P, Langer HF. Platelets in inflam- mation and atherogenesis. Front Immunol 2015;6:98
21. Kim CH, Kim SJ, Lee MJ, Kwon YE, Kim LY, Park KS, et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS One 2015;10:e0119437
Google Scholar
22. Yusuf I, Agusni I. Lymphocyte response to mycobacterium leprae antigens in reversal reaction state of leprosy. Indones J Trop Infect Dis 2015;5:96
Google Scholar
Congratulatory MessageClick here!