pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Ye-Jin Lee,Kyung Sook Kim,Hye-Jin Ahn,Min Kyung Shin
10.17966/JMI.2024.29.2.48 Epub 2024 July 07
Abstract
Background: Nonthermal atmospheric plasma (NTAP) has been studied as a new treatment option for various medical conditions. Onychomycosis is a common nail disorder caused by a fungus that is difficult to treat and often recurs. One reason for treatment difficulty is the growing resistance to current antifungal medications. Given the medical concerns related to antifungal drugs, there is a need for newly developed treatment options.
Objective: This study sought to determine if NTAP was effective as an antifungal treatment for onychomycosis. To explore this innovative treatment option, we conducted in vitro and ex vivo experiments using microbial methods and microscopy.
Methods: For the in vitro experiments, Trichophyton (T.) rubrum was inoculated into Sabouraud dextrose agar plates. For the ex vivo experiments, nail specimens diagnosed with onychomycosis resulting from T. rubrum were used. NTAP using argon gas was utilized in this study. Scanning electron and reflectance confocal microscopy were used to analyze the nail specimens.
Results: The growth of T. rubrum colonies exposed to NTAP was markedly suppressed compared to untreated colonies in vitro. T. rubrum ultra-structures noticeably transformed from smooth to rough or ruptured after exposure.
Conclusion: NTAP can inhibit the growth of T. rubrum and cause damage to fungal spores. This finding could potentially lead to the development of new and effective treatments for onychomycosis.
Keywords
Non-thermal atmospheric plasma Onychomycosis Plasma Reflectance confocal microscopy Scanning electron microscope Trichophyton rubrum
Onychomycosis is the most common infectious nail disorder, with a reported prevalence of approximately 5.5% in the general population1. This disease is commonly caused by fungal infections, dermatophytes, especially Trichophyton (T.) rubrum, nondermatophyte molds (NDMs), and yeasts. Recently, bacteria-fungal coinfections and fungal biofilms in onychomycosis have been discovered, leading to difficulty in treating onychomycosis2-5. It has also been reported that resistance to antifungal agents is increasing6.
Plasma is the fourth state of matter comprising electro- magnetic radiation, excited gas, ions, reactive species, and free radicals. One of the well-known effects of plasma is its ability to eliminate bacteria, affording it antimicrobial effects7. However, previous studies have shown that plasma also has antifungal properties8-11. In particular, the nonthermal atmo- spheric plasma (NTAP) has demonstrated its ability to kill clinically important fungal species like T. rubrum, Microsporum canis, and Candida albicans in vitro8. Recent studies have also shown the effectiveness of NTAP in treating fungal infections using a human nail model. In this study, we aim to evaluate the efficacy of NTAP in treating T. rubrum and analyze the ultrastructural changes in the fungus before and after NTAP treatment11,12.
In vitro experiments were conducted to assess the effect- iveness of NTAP against fungal infections and to determine the optimal duration of fungal inhibition required for ex vivo experiments.
1. T. rubrum strain and culturing in vitro
In this in vitro study, a fungal strain called T. rubrum was isolated from patients who visited our clinic and gave informed consent. The patients were diagnosed with onychomycosis, tinea pedis, or tinea cruris through mycological examination, such as potassium hydroxide (KOH) smear, fungal culture, and periodic acid-Schiff staining. The strain was confirmed to be T. rubrum through fungal culture. The isolate was then inoculated on Sabouraud dextrose agar (SDA) plates and incubated at 30℃ for a week.
Twelve samples were taken using an 8 mm punch biopsy instrument and transplanted into four new SDA plates, with three colonies per plate. The colonies were then exposed to NTAP jet plasma for 0, 10, and 30 minutes (as shown in Fig. 1), and photographs were taken daily with a digital camera (Canon EOS 80D). The colony sizes were measured using Image J software to assess their growth.

2. Ex vivo nail specimens
Nail samples were collected from twelve patients with in- grown nails or onychogryphosis combined with onychomycosis. After obtaining informed consent from the patients, the nails were partially removed for medical purposes (Institutional Review Board of KHUH 2020-09-034). The nails were exam- ined through mycological studies using techniques like KOH smear and fungal culture. The samples infected with T. rubrum were included in the study. Each nail sample was divided into two parts. One part was left untreated, while the other was exposed to plasma for 30 minutes.
3. NTAP irradiation
We utilized NTAP generated using argon gas with 99.99% purity at 40℃. The argon gas flow rate was a constant 1.5 liters per minute (lpm) during NTAP generation. The specimens were placed 3 mm away from the bottom of the NTAP flame and were directly exposed for either 10 or 30 minutes.
4. Scanning electron microscopy (SEM)
To analyze the morphological changes in fungi, the samples of fungal colonies and nail specimens were prepared as done in previous studies13,14. The samples were fixed with 4% paraformaldehyde and 2.5% glutaraldehyde at 4℃. They were then washed with 0.1 M sodium cacodylate buffer (pH 7.4) for 10 min. The subsequent procedures were performed using an automatic tissue processing device. Specimens were fixed with osmium tetroxide for 2 h, cleaned in distilled water for 2 × 10 min, and dehydrated subsequently in 50%, 70%, 90%, and 100% ethanol for 10 min each, followed by washing in solutions with 1:1, 3:7, and 1:9 ratios of alcohol: acetic acid for 10 min each, pure isoamyl acetate for 10 min, and subsequently dried in hexamethyldisilane amine dioxane. The samples were observed under a field-emission scanning electron microscope (Inspect F, FEI, USA) before and after NTAP.
5. Reflectance confocal microscopic (RCM) examination
Ex vivo morphological changes of fungal nail specimens were analyzed with RCM (VivaScope○R 1500, Lucid Inc., Rochester, NY, USA) before and after NTAP irradiation at a laser power below 5.8~6.0 watts.
6. Statistical analysis
The colony sizes obtained from the in vitro study were analyzed using the Kruskal-Wallis and Mann-Whitney tests with SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA) at a significance level of p<0.05.
1. In vitro: The change of colony sizes after NTAP irradiation in vitro
The study's results are presented in Fig. 2, showing the median colony sizes between days 0 and 7 after exposure to NTAP irradiation for different durations. The initial colony size was approximately 0.503 cm2. Compared to the control group that was not irradiated, the size of the colonies exposed to 10 minutes of irradiation was reduced to 0.49 cm2 on day 2. Still, the colony sizes gradually increased after day 2. Colonies exposed to 30 minutes of irradiation demonstrated progressive reductions in size from days 1 to 7, resulting in significantly reduced sizes on day 7 (0.421 cm2). When com- paring the macroscopic morphologies of colonies, it was observed that the peripheral growth zone was destroyed in the 30 min-irradiated colonies. In contrast, the peripheral zone was visible in the control colony (Fig. 2).

The structure of T. rubrum was examined using SEM, as shown in Fig. 3. In the untreated T. rubrum sample, smooth-surfaced, long, and branching hyphae were observed, along with a biofilm covering the hyphae (Fig. 3A). The hyphae of the T. rubrum sample treated with NTAP shrank and cracked (Fig. 3B). After 30 minutes of NTAP irradiation, the hyphae structures appeared severely damaged; no smooth-surfaced, long hyphae or biofilm structures were detected (Fig. 3C).
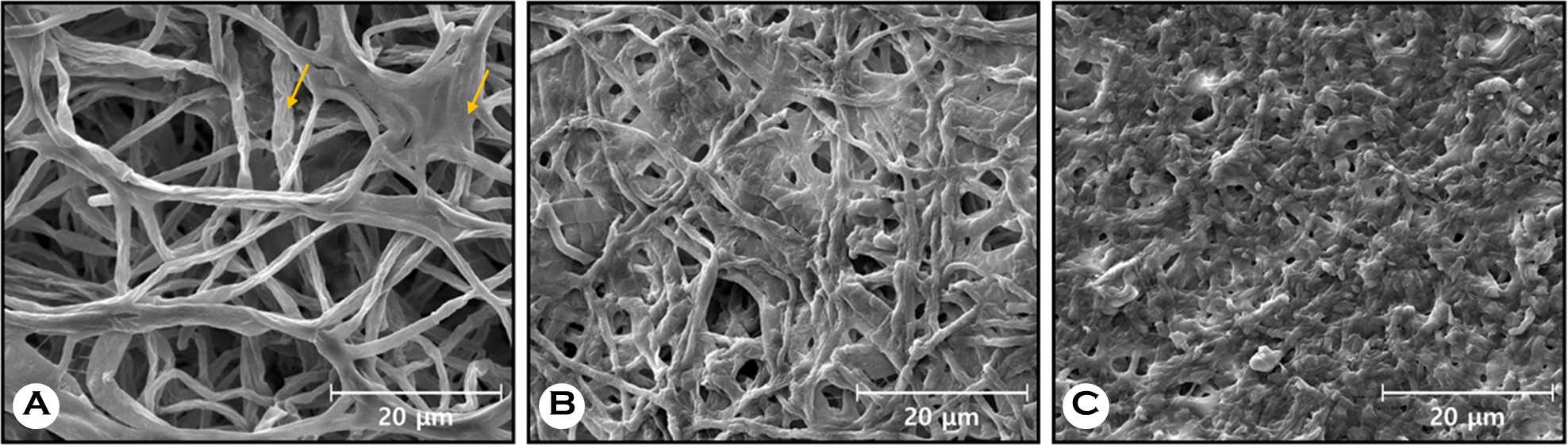
2. Ex vivo: Effects of NTAP irradiation on the ultrastructure of fungal infected nails by using SEM
SEM was used to examine nail specimens before and after NTAP irradiation. A total of 362 images from 12 infected nails were analyzed. Fig. 4A and D show images of untreated nails. Long and intact hyphae structures, along with numerous rod-shaped bacteria, were observed (Fig. 4A). Fungal spores with smooth globular structures were also observed (Fig. 4D). After NTAP exposure for 30 minutes, the structure of hyphae changed to rough and short, and the rod-shaped bacteria around the hyphae disappeared (Fig. 4B and C). Several fungal spores ruptured and collapsed (Fig. 4E).

The cross-section of the nail plate under SEM revealed that the porosity increased after NTAP exposure (Fig. 5B) compared to the untreated nail (Fig. 5A).

3. Use of RCM to evaluate the effects of NTAP irradiation on nail ultrastructure in nails with fungal infections
Long filamentous hyphal structures and spherical fungal spores were identified in the infected nails (as seen in Fig. 6A and C). Post-NTAP irradiation, the hyphal structures shortened and burst, and grouped fungal spores scattered (as seen in Fig. 6B and D).

NTAP has attracted increasing interest as a possible treat- ment for infectious diseases15,16. We applied NTAP to T. rubrum, the most common cause of fungal skin infections. The results showed that NTAP inhibited fungal growth in vitro and induced morphological changes in the fungus in vitro and ex vivo.
Importantly, fungal growth inhibition depended on plasma exposure duration. Although a 10-minute exposure initially proved sufficient to prevent growth, after 7 days, NTAP ex- posure for 10 minutes had a similar effect to that of the untreated control. In contrast, a 30-minute exposure demon- strated a significant fungistatic effect. Moreover, the dose-dependent fungistatic effect was confirmed by the structural changes observed under SEM in vitro (as shown in Fig. 4).
Further examination of T. rubrum or nail specimens in vivo and ex vivo, respectively, under SEM and RCM after NTAP irradiation, showed that the hyphae changed from long and smooth to rough and bursting, the spores ruptured, and the fungal biofilm was destroyed. This suggests that NTAP has a fungistatic and even fungicidal effect in vitro and ex vivo.
Although there have been many hypotheses about the mechanisms of biological effects of NTAP, some researchers have postulated an association with oxidative damage caused by reactive species, such as reactive oxygen or nitrogen species, generated as a product of plasma interactions with the surrounding media15,17-19. Therefore, it is hypothesized that the antifungal effects of NTAP are synergistically attributed to (1) oxidative damage caused by reactive species and (2) partial mechanical damage to fungus, based on the micro- scopic changes in fungal structures we observed.
NTAP can enhance drug permeation, as shown in the larger size and number of pores observed in the plasma-exposed nails (Fig. 5). Nails are composed of keratins with disulfide bridges that act as major barriers against drug per- meation20,21. A study by Gómez and colleagues revealed that nails affected by onychomycosis had increased porosity and decreased disulfide bonds compared to healthy nails20. Fig. 5 of the study displayed untreated nails with several pores in the cross-section, while nail plate porosity increased after NTAP irradiation20. Borges et al. analyzed the impact of NTAP on the keratin structure of nail surfaces using attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR)11. Plasma exposure increases porosity, improving drug delivery. NTAP aids percutaneous drug absorption22,23. although the exact mechanism remains unknown. However, research on skin porosity after plasma exposure suggests that pore formation is similar to electroporation or results from changes in lipid structures under an electric field after plasma. The pores generated can facilitate drug permeation. There- fore, NTAP may also affect topical antifungal and systemic treatments.
NTAP could be safer than systemic treatments for treating onychomycosis, which can cause liver failure and compliance issues in some patients, including the elderly1.
In a pilot study conducted by Lipner et al.24, NTAP was used to treat onychomycosis. The study showed that NTAP was relatively safe and effective, with a clinical cure rate of 53.8% observed in 13 patients. A few studies used NTAP to treat oral Candida species. Zamperini et al.25 demonstrated that plasma exposure could modify the surface of the resin and reduce Candida glabrata adhesion in vitro. Preissner et al.26 conducted a pilot study that found NTAP could be used as an adjuvant antifungal therapy for oral candidiasis. There- fore, the antifungal effects of NTAP are demonstrated against onychomycosis by T. rubrum and onychomycosis caused by other fungal pathogens. Additionally, NTAP destroyed bacteria and fungi, making it helpful in treating onychomycosis with bacterial coinfection3.
This research was conducted to validate the effectiveness of NTAP against T. rubrum, a dermatophyte. However, further studies on other causal species are required. Nonetheless, SEM and RCM allowed for observing structural changes in real-time, confirming that NTAP causes physical damage to the fungal surfaces and spores. NTAP can eliminate fungal biofilms known to cause stubborn onychomycosis. Biofilms are organized microbial communities that adhere to surfaces and each other through an extracellular polymeric matrix and can be verified by SEM images27. Several studies have demon- strated the antibiofilm properties of NTAP for bacteria28,29, and this research has shown efficacy against fungus.
In summary, NTAP irradiation inhibited the growth of T. rubrum, and structural damage worsened as the dose in- creased. NTAP may be an alternative therapy to treating drug-resistant infections and enhancing the therapeutic effects of antifungal drugs.
References
1. Lipner SR, Scher RK. Onychomycosis: Clinical overview and diagnosis. J Am Acad Dermatol 2019;80:835-851
Google Scholar
2. Foster KW, Thomas L, Warner J, Desmond R, Elewski BE. A bipartite interaction between Pseudomonas aeruginosa and fungi in onychomycosis. Arch Dermatol 2005;141: 1467-1468
Google Scholar
3. Yang YS, Ahn JJ, Shin MK, Lee MH. Fusarium solani onychomycosis of the thumbnail coinfected with Pseudo- monas aeruginosa: Report of two cases. Mycoses 2011; 54:168-171
Google Scholar
4. Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Crit Rev Microbiol 2009;35:340-355
Google Scholar
5. Fanning S, Mitchell AP. Fungal biofilms. PLoS Pathog 2012;8:e1002585
6. Gupta AK, Renaud HJ, Quinlan EM, Shear NH, Piguet V. The growing problem of antifungal resistance in onycho- mycosis and other superficial mycoses. Am J Clin Dermatol 2021;22:149-157
Google Scholar
7. Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia LH. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm 2001;226:1-21
Google Scholar
8. Daeschlein G, Scholz S, von Woedtke T, Niggemeier M, Kindel E, Brandenburg R, et al. In vitro killing of clinical fungal strains by low-temperature atmospheric-pressure plasma jet. IEEE Trans Plasma Sci 2010;39:815-821
Google Scholar
9. Heinlin J, Maisch T, Zimmermann JL, Shimizu T, Holzmann T, Simon M, et al. Contact-free inactivation of Trichophyton rubrum and Microsporum canis by cold atmospheric plasma treatment. Future Microbiol 2013;8:1097-1106
Google Scholar
10. Shemer A, Daniel R, Kassem R, Geffen Y, Galili E. Cold sub-atmospheric and atmospheric pressure plasma for the treatment of Trichophyton rubrum onychomycosis: An in-vitro study. Dermatol Ther 2020;33:e14084
Google Scholar
11. Borges AC, Nishime TMC, de Moura Rovetta S, Lima GMG, Kostov KG, Thim GP, et al. Cold atmospheric pres- sure plasma jet reduces Trichophyton rubrum adherence and infection capacity. Mycopathologia 2019;184:585-595
Google Scholar
12. Bulson JM, Liveris D, Derkatch I, Friedman G, Geliebter J, Park S, et al. Non-thermal atmospheric plasma treat- ment of onychomycosis in an in vitro human nail model. Mycoses 2020;63:225-232
Google Scholar
13. Yue X, Li Q, Wang H, Sun Y, Wang A, Zhang Q, et al. An ultrastructural study of Trichophyton rubrum induced onychomycosis. BMC Infect Dis 2015;15:532
Google Scholar
14. Yue X, Li Q, Wang H, Sun Y, Wang A, Zhang Q, et al. Scanning electron microscopy of the nail plate in onycho- mycosis patients with negative fungal culture. Scanning 2016;38:172-176
Google Scholar
15. Gan L, Jiang J, Duan JW, Wu XJZ, Zhang S, Duan XR, et al. Cold atmospheric plasma applications in dermatology: A systematic review. J Biophotonics 2021;14:e202000415
Google Scholar
16. Gan L, Zhang S, Poorun D, Liu D, Lu X, He M, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges 2018;16: 7-13
Google Scholar
17. Winter T, Winter J, Polak M, Kusch K, Mäder U, Sietmann R, et al. Characterization of the global impact of low temperature gas plasma on vegetative microorganisms. Proteomics 2011;11:3518-3530
Google Scholar
18. Winter T, Bernhardt J, Winter J, Mäder U, Schlüter R, Weltmann KD, et al. Common versus noble Bacillus sub- tilis differentially responds to air and argon gas plasma. Proteomics 2013;13:2608-2621
Google Scholar
19. Bourke P, Ziuzina D, Han L, Cullen PJ, Gilmore BF. Micro- biological interactions with cold plasma. J Appl Microbiol 2017;123:308-324
Google Scholar
20. Cutrín Gómez E, Anguiano Igea S, Delgado-Charro MB, Gómez Amoza JL, Otero Espinar FJ. Microstructural alter- ations in the onychomycotic and psoriatic nail: Relevance in drug delivery. Eur J Pharm Biopharm 2018;128:48-56
Google Scholar
21. Baswan S, Kasting GB, Li SK, Wickett R, Adams B, Eurich S, et al. Understanding the formidable nail barrier: A review of the nail microstructure, composition and dis- eases. Mycoses 2017;60:284-295
Google Scholar
22. Lademann O, Richter H, Meinke MC, Patzelt A, Kramer A, Hinz P, et al. Drug delivery through the skin barrier enhanced by treatment with tissue-tolerable plasma. Exp Dermatol 2011;20:488-490
Google Scholar
23. Gelker M, Müller-Goymann CC, Viöl W. Permeabilization of human stratum corneum and full-thickness skin samples by a direct dielectric barrier discharge. Clinical Plasma Medicine 2018;9:34-40
Google Scholar
24. Lipner SR, Friedman G, Scher RK. Pilot study to evaluate a plasma device for the treatment of onychomycosis. Clin Exp Dermatol 2017;42:295-298
Google Scholar
25. Zamperini CA, Carneiro Hde L, Rangel EC, Cruz NC, Vergani CE, Machado AL. In vitro adhesion of Candida glabrata to denture base acrylic resin modified by glow-discharge plasma treatment. Mycoses 2013;56:134-144
Google Scholar
26. Preissner S, Kastner I, Schütte E, Hartwig S, Schmidt-Westhausen AM, Paris S, et al. Adjuvant antifungal therapy using tissue tolerable plasma on oral mucosa and removable dentures in oral candidiasis patients: a randomised double-blinded split-mouth pilot study. Mycoses 2016;59:467-475
Google Scholar
27. Costa-Orlandi CB, Sardi JC, Santos CT, Fusco-Almeida AM, Mendes-Giannini MJ. In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling 2014;30:719-727
Google Scholar
28. Lee MH, Park BJ, Jin SC, Kim D, Han I, Kim J, et al. Removal and sterilization of biofilms and planktonic bacteria by microwave-induced argon plasma at atmospheric pressure. New J Phys 2009;11:115022
Google Scholar
29. Paldrychová M, Vaňková E, Kašparová P, Sembolová E, Maťátková O, Masák J, et al. Use of non-thermal plasma pre-treatment to enhance antibiotic action against mature Pseudomonas aeruginosa biofilms. World J Microbiol Biotechnol 2020;36:108
Congratulatory MessageClick here!