pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Jin Ho Kim,Yong Jun Bang,Jae Bok Jun,Weon Ju Lee
10.17966/JMI.2023.28.3.79 Epub 2023 October 06
Abstract
Purpureocillium lilacinum is a saprophytic fungus with a ubiquitous environmental distribution. Unfortunately, no standard treatment has yet been established for cutaneous P. lilacinum infections. Based on the in vitro antifungal susceptibility test, posaconazole has been considered an effective treatment option. We herein present a case involving a 72-year-old woman who visited our clinic due to a peripherally spreading, well-demarcated, asymptomatic, scaly, and erythematous patch on her forehead that had persisted for 4 months. She had been diagnosed with cutaneous P. lilacinum infection and had been treated with itraconazole (200 mg/day). However, the lesion recurred in the same area. Histopathological findings revealed suppurative granulomatous dermatitis with fungal elements. Fungal culture confirmed P. lilacinum regrowth. Posaconazole was selected to treat the recurrence of P. lilacinum infection. After 10 weeks of treatment, the lesion decreased dramatically without any adverse drug events. We recommend posaconazole as a treatment option for P. lilacinum infection refractory to itraconazole.
Keywords
Cutaneous Posaconazole Purpureocillium lilacinum Refractory to itraconazole
Purpureocillium lilacinum, a ubiquitous mold found in soil and vegetation, has emerged as a pathogen in immuno- competent and immunocompromised patients1. Although no standard antifungal regimen has been established for P. lilacinum, it has been known for possessing resistance to typical antifungals, such as amphotericin B, fluconazole, and flucytosine. Therefore, P. lilacinum infections are hard to manage given their frequent recurrence with conventional antifungal agents. Nevertheless, several cases have shown that monotherapy or combination therapy with itraconazole, ketoconazole, terbinafine, and surgical debridement can be used to treat cutaneous P. lilacinum infections. Given the con- flicting results of in vitro tests for susceptibility to itraconazole, recurrence or treatment failure has also often been reported with itraconazole treatment for P. lilacinum2. Therefore, new second-generation triazole antifungals, including voriconazole, posaconazole, and isavuconazole, have been used to treat cutaneous P. lilacinum infections.
Posaconazole (Noxafil®) had been approved by the Food and Drug Agency (FDA) in 2005 for the treatment of inva- sive aspergillosis and candida infections. However, emerging studies have shown that the effects of posaconazole can extend to various fungal and mold infections3. Herein, we report the use of posaconazole in a patient with cutaneous P. lilacinum infection who failed to achieve complete remission with itraconazole in our last report2.
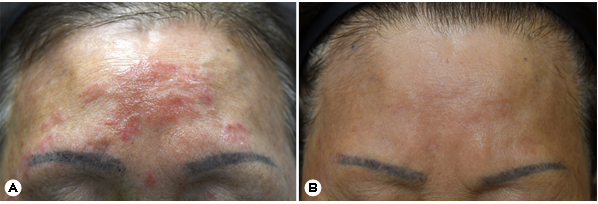
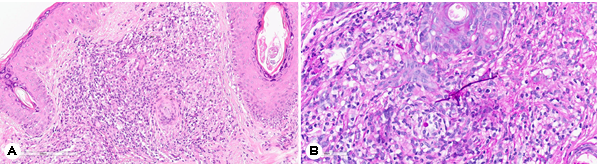
A 72-year-old female presented with an asymptomatic, peripherally spreading, well-demarcated, scaly, and erythema- tous patch on her forehead that had persisted for 4 months (Fig. 1A). Approximately 2 years prior to presentation, she was been diagnosed with a subcutaneous P. lilacinum infection confirmed through skin biopsy, fungal culture, and sequence analysis of the internal transcribed spacer region of the rRNA gene. She had been treated with oral itraconazole (200 mg/day) for 4 months, with most of the skin lesions improving after 4 months of treatment. However, 2 months after ceasing itraconazole, the lesion recurred in her forehead. Despite repeated use of itraconazole for each recurrence, complete remission could not be achieved. Skin biopsy and fungal culture were then conducted. Hematoxylin and eosin staining revealed superficial, interstitial, and perifollicular lympho- histiocytic infiltration and granulomatous inflammation with multinucleated giant cells (Fig. 2A). Periodic acid-Schiff staining with diastase revealed fungal elements and mixed inflam- matory and granulomatous infiltration in the dermis (Fig. 2B). Matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis identified P. lilacinum. The patient was then prescribed oral posaconazole (100 mg/day) for 10 weeks. Her skin lesions had dramatically decreased after 10 weeks of treatment, with no adverse events having been reported during this period (Fig. 1B). Approximately 2 months after stopping posaconazole, her lesions were entirely resolved without any recurrence.
P. lilacinum causes a variety of clinical manifestations in immunocompetent and immunocompromised patients, ranging from superficial mycoses to life-threatening systemic infections4. Thus far, the Korean literature has reported nine cases of cutaneous P. lilacinum infections2. Five of the cases were successfully treated with itraconazole monotherapy. However, two cases showed resistance to itraconazole and required alternative therapy with voriconazole or terbinafine. Our patient also experienced treatment failure with itracona- zole2. Therefore, alternative antifungal agents are necessary for the treatment of cutaneous P. lilacinum infections.
Although a standard treatment guideline has yet to be established, new azoles, such as voriconazole, posaconazole, and isavuconazole, have been used to treat cutaneous P. lilacinum infections. In fact, two recently reported guidelines5,6 have moderately recommended voriconazole and marginally recommended posaconazole for the treatment of P. lilacinum infections.
However, we recommend using posaconazole as the first alternative therapeutic option in P. lilacinum infections refrac- tory to itraconazole given its potentially better effectiveness and safety. First, we assert that posaconazole could be more effective than voriconazole in treating P. lilacinum infection. The effectiveness of posaconazole for P. lilacinum infections can be implied from the results of the antifungal susceptibility test. Although many cases have reported successful treat- ment of cutaneous P. lilacinum infections with itraconazole, itraconazole showed only intermediate antifungal activity against P. lilacinum. Overall, posaconazole showed the best in vitro activity against P. lilacinum, with MICs ≤0.5 mg/L. In addition, posaconazole was effective at a lower range (0.06~ 0.5 mg/L) than voriconazole (0.06~4 mg/L). Therefore, posa- conazole could more effectively treat P. lilacinum infections than voriconazole.
Moreover, posaconazole could be safer than voriconazole due to fewer treatment-related adverse events. A controlled study comparing posaconazole with voriconazole for the treatment of invasive aspergillosis by Maertens et al.8 revealed that the overall incidence of treatment-related adverse event rates was 30% for posaconazole and 40% for voriconazole. Also, the American FDA suggests that isavuconazole and posaconazole are better tolerated than voriconazole with fewer neurological disorders (hallucinations, peripheral neuro- pathies), skin toxicities, and interactions with immunosup-pressive treatment9.
Nowadays, studies have reported the successful treatment of cutaneous P. lilacinum infections with voriconazole and posaconazole. In fact, over 30 cases of voriconazole use and 9 cases of posaconazole use have been reported for cutaneous /subcutaneous P. lilacinum infections9-16. We reviewed the demographic characteristics of these cases, including their age, sex, relevant history, treatment, complications related to posaconazole, and outcomes (Table 1). Seven of the nine cases were associated with underlying immunosuppression, including organ transplantation, Evans's syndrome, and long-term use of systemic corticosteroids. Six cases first used other antifungal agents, such as itraconazole and voriconazole. In particular, two cases who failed to respond to voriconazole treatment for P. lilacinum infection were subsequently cured after posaconazole treatment. One case could not continue using voriconazole because of adverse events.
|
Authors |
Age/ |
Risk factors |
Initial |
Secondary |
Complication |
Outcome |
|
Albert et al.9 |
52/F |
Hepatoreanl |
Posaconazole |
Surgical |
Digestive disorder |
Remission |
|
Chen et al.10 |
40/M |
Evan's syndrome |
Voriconazole |
Posaconazole |
None |
Remission |
|
Ezzedine et al.11 |
60/M |
Rheumatoid |
Voriconazole |
Posaconazole |
Anorexia, involuntary |
Improvement |
|
Hu et al.12 |
63/F |
Evan's syndrome |
Posaconazole |
None |
AST/ALT elevation |
Remission |
|
Martinez et al.13 |
59/M |
Heart |
Posaconazole |
None |
None |
Remission |
|
McGeachie et al.14 |
53/F |
None |
Voriconazole |
Posaconazole |
None |
Remission |
|
Paul et al.15 |
68/M |
Kidney |
Itraconazole |
Posaconazole |
None |
Remission |
|
Xia et al.16 |
53/M |
Autoimmune |
Itraconazole |
Posaconazole |
None |
Remission |
|
Our case |
72/F |
None |
Itraconazole |
Posaconazole |
None |
Remission |
Posaconazole is often connected with the following adverse drug reactions: neutropenia, electrolyte dysfunction, head- ache, vomiting, nausea, stomachache, diarrhea, dyspepsia, and hepatic disturbances including elevation of alanine transaminase (ALT) and aspartate aminotransferase (AST)17. Physicians need to carefully monitor the electrolyte levels of patients, including hypokalemia and hepatic function, through- out the course of therapy. Nevertheless, all of these adverse drug reactions, except for neutropenia, can be managed by reducing the dose or adding histamine-2 blockers or proton pump inhibitors. In the case reported by Hu et al.12, the patient showed elevated AST and ALT levels during oral posaconazole treatment. The plasma concentration of posa- conazole was 3.04 μg/mL, whereas its MIC in vitro was 1 μg /mL. After reducing the dosage of posaconazole to 200 mg /day, the patient's AST and ALT levels normalized, with a considerable decrease in her skin lesions also having been observed. In our case, we showed that posaconazole had a sufficient effect on cutaneous P. lilacinum infection without elevating AST and ALT levels despite using a posaconazole dose (100 mg/day) lower than that reported in other case studies. Given that posaconazole is often accompanied by gastric disturbances, famotidine (20 mg/day) can be added as a preventive measure. Our patient did not show any adverse reactions to posaconazole during treatment. Taken together, our experience suggests that the safety of posaconazole can be more reliable and easily managed.
In conclusion, we report a case involving a patient who developed recurrence of cutaneous P. lilacinum infection after itraconazole treatment. Posaconazole exhibited sub- stantial efficacy and good tolerability in this case. We believe posaconazole can be a therapeutic option for cutaneous P. lilacinum infections refractory to itraconazole.
References
1. Luangsa-Ard J, Houbraken J, van Doorn T, Hong SB, Borman AM, Hywel-Jones NL, et al. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol Lett 2011;321:141-149
Google Scholar
2. Ha NG, Park KD, Bang YJ, Jun JB, Choi JS, Lee WJ. A case of cutaneous Purpureocillium lilacinum infection looking like psoriasis. J mycol Infect 2021;26:72-76
Google Scholar
3. Leung S, Poulakos MN, Machin J. Posaconazole: An update of its clinical use. Pharmacy 2015;3:210-268
Google Scholar
4. Sprute R, Salmanton-Garcia J, Sal E, Malaj X, Racil Z, Ruiz de Alegria Puig C, et al. Invasive infections with Purpureo- cillium lilacinum: clinical characteristics and outcome of 101 cases from FungiScope® and the literature. J Anti- microb Chemother 2021;76:1593-1603
Google Scholar
5. Bupha-Intr O, Butters C, Reynolds G, Kennedy K, Meyer W, Patil S, et al. Consensus guidelines for the diagnosis and management of invasive fungal disease due to moulds other than Aspergillus in the haematology/oncology setting, 2021. Intern Med J 2021;51:177-219
Google Scholar
6. Hoenigl M, Salmanton-Garcia J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis 2021;21:e246-e257
Google Scholar
7. Monpierre L, Aït-Ammar N, Valsecchi I, Normand AC, Guitard J, Riat A, et al. Species identification and in vitro antifungal susceptibility of Paecilomyces/Purpureocillium species isolated from clinical respiratory samples: A multi- center study. J Fungi 2022;8:684
8. Martens JA, Rahav G, Lee DG, Ponce-de-Leon A, Ramírez Sánchez IC, Klimko N, et al. Posaconazole versus vori- conazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non-inferiority trial. Lancet 2021;397:499-509
Google Scholar
9. Albert R, Lemaignen A, Desoubeaux G, Bailly E, Bernard L, Lacasse M. Chronic subcutaneous infection of Purpureo- cillium lilacinum in an immunocompromised patient: Case report and review of the literature. Med Mycol Case Rep 2022;38:5-8
Google Scholar
10. Chen WY, Lin SR, Hung SJ. Successful treatment of re- current cutaneous Purpureocillium lilacinum (Paeclomyces lilacinus) infection with posaconazole and surgical de- bridement. Acta Derm Venereol 2019;99:1313-1314
Google Scholar
11. Ezzedine K, Belin E, Guillet S, D'Almeida M, Droitcourt C, Accocebery I, et al. Cutaneous hyphomycosis due to Paecilomyces lilacinus. Acta Derm Venereol 2012;92: 156-157
Google Scholar
12. Hu X, Zhang L, Lin Q, Zhang F, Zhao X. Complicated multiple organ infection of Purpureocillium lilacinum and varicella-zoster virus infection in a patient with Evans' syndrome. Blood Sci 2022;4:89-92
Google Scholar
13. Martinez E, Vandergriff T, Vasquez R. Cutaneous Paecilo- myces infection in an immunocompromised patient in the setting of postthrombotic syndrome successfully treated with posaconazole. JAAD Case Rep 2020;6:1144-1146
Google Scholar
14. McGeachie DL, Boyce AE, Miller RM. Recurrent cutaneous hyalohyphomycosis secondary to Purpureocillium lilacinum in an immunocompetent individual. Australas J Dermatol 2021;62:e411-e413
Google Scholar
15. Paul J, Czech MM, Balijepally R, Brown JW. Diagnostic and therapeutic challenges of treating opportunistic fungal cellulitis: a case series. BMC Infect Dis 2022;22: 435
Google Scholar
16. Xia XJ, Liu ZH. Cutaneous hyalophomycosis caused by Purpureocillium lilacinum in an autoimmune hemolytic anemia patient with previous pulmonary tuberculosis and cutaneous cryptococcosis infection. Mycopathologia 2021;186:563-564
Google Scholar
17. Sienkiewicz BM, Łapiński Ł, Wiela-Hojenska A. Com- parison of clinical pharmacology of voriconazole and posaconazole. Contemp Oncol 2016;20:365-373
Congratulatory MessageClick here!