pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Yi Na Yoon,Ji Won Lim,Hyung Seok Son,Young Ah Cho,Yang Won Lee,Yong Beom Choe,Da-Ae Yu
10.17966/JMI.2023.28.3.74 Epub 2023 October 06
Abstract
Disseminated coccidioidomycosis is an uncommon fungal infection primarily observed in immunocompromised individuals. Infection typically occurs when individuals inhale fungal spores in endemic areas such as the southwestern United States and parts of Central and South America. However, globalization has increased the number of reported cases outside endemic areas. Herein, we present an unusual case of coccidioidal meningitis, a rare but fatal complication of disseminated coccidioidomycosis, in an immunocompetent patient who previously resided in an endemic area.
Keywords
Coccidioidal meningitis Coccidioidomycosis
Coccidioidomycosis, also known as Valley Fever, is a dimor- phic fungal infection caused by Coccidioides immitis and Coccidioides posadasii. These species are present in either a mycelium state or a spherule state. In natural environments such as soil, the organism exhibits mycelial growth through apical extension. When the soil is disturbed by natural pro- cesses or human activities, the spores (known as arthro- conidia) can become airborne, potentially causing illness when inhaled. It is endemic to the southwestern United States, as well as to some regions of Mexico, Central America, and South America1. Most coccidioidal infections are asymptomatic; however, in approximately one-third of cases, it progresses to pulmonary infection and represents a major cause of community-acquired pneumonia in areas with a high disease prevalence. Coccidioidomycosis rarely manifests as extra- pulmonary infections of the skin, bones, joints, or central nervous system2. Disseminated coccidioidomycosis is com- monly observed in older adults and patients with immuno- suppressive conditions (such as AIDS and cancer) or those receiving immunosuppressive drugs2. Herein, we report an unusual case of coccidioidal meningitis in a young, immuno- competent patient in Korea.
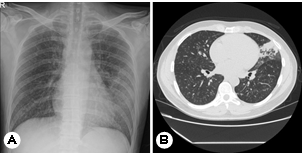
A 35-year-old man presented with a two-month history of fever, headache, cough, and an erythematous plaque with grouped vesicles on the left side of his forehead (Fig. 1). This patient, a resident of Los Angeles, USA, often jogged in dusty environments. The patient returned to Korea for treatment owing to the persistence of severe headaches and cough. Peripheral blood test results were as follows: white blood cell (WBC) count: 11,700/mm3 (neutrophils: 45%; lymphocytes: 30%; monocytes: 3%; eosinophils: 15%; basophils: 1%): hemoglobin: 14.4 g/dL; platelets: 414:000/ mm3; and high-sensitivity C-reactive protein (HS-CRP): 4.82 mg /mm3. Chest radiography findings indicated haziness in the left and right lower lungs, and chest computed tomography (CT) scans revealed multiple tiny nodules, consolidation in both lungs, and lymphadenopathy (Fig. 2). Tuberculosis was suspected; therefore, treatment with antituberculosis medi- cations was initiated. However, his fever persisted, and blood test results indicated eosinophilia; therefore, the patient was hospitalized for further workup. Polymerase chain reaction analyses performed after hospitalization were negative for tuberculosis, and no other respiratory pathogens were de- tected. Furthermore, bacteria, fungi, and Mycobacterium tuberculosis were not identified via culture.
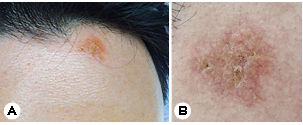
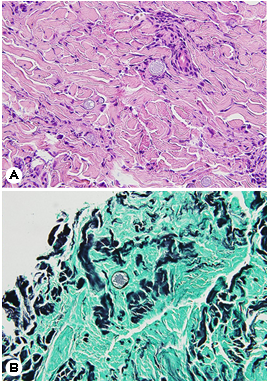
The patient was referred to the Department of Derma- tology for a skin lesion biopsy. An erythematous plaque was identified on the left side of his forehead, and, according to the patient, clustered vesicles had appeared and disappeared repeatedly two months earlier. Under the clinical impression of lupus vulgaris, sarcoidosis, or herpes simplex virus infection, a 3-mm punch biopsy was performed. Histological exam- ination results revealed pseudoepitheliomatous hyperplasia in the upper epidermis and thick-walled spherules surrounded with exudative granulomas, suggesting coccidioidomycosis (Fig. 3). The patient was subsequently diagnosed with dis- seminated coccidiomycosis involving the skin.
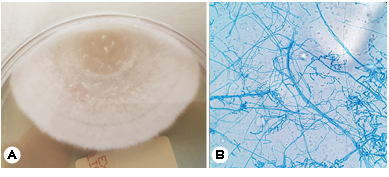
The patient's respiratory symptoms worsened, and bron- choscopy and bronchoalveolar lavage were performed. The differential leukocyte count revealed an increase in the neutrophil count to 79.3%, and Coccidioides species were identified in the fungal culture (Fig. 4). The patient sub- sequently complained of hiccups and numbness in his left limbs, and loss of consciousness was observed after his headache and vomiting had worsened. The patient was transferred to the intensive care unit, and spinal tapping was performed. His cerebrospinal fluid (CSF) examination results were as follows: WBC count: 631/μL; protein: 70.1 mg/dL; and glucose: 38 mg/dL, and brain magnetic resonance imaging (MRI) revealed hydrocephalus in both lateral ventricles (Fig. 5). Emergency external ventricular drain (EVD) placement was performed.
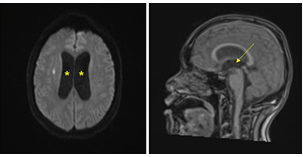
In the CSF, the Coccidioides antigen and antibody test results were negative, whereas the serum antigen and anti- body test results were positive. Subsequent whole-genome sequencing conducted for species identification confirmed it as Coccidioides immitis. Consequently, the patient was diagnosed with coccidioidal meningitis and treated with intravenous fluconazole (1,200 mg/day). Despite the admin- istration of fluconazole and EVD, the hydrocephalus did not improve. After three weeks of treatment, the patient was administered voriconazole; however, his overall condition, including hyponatremia and pneumonia, deteriorated. Intra- venous amphotericin and high-dose steroids with antibiotics were administered but the systemic infection did not improve, and the patient died due to disseminated intravascular coagu- lation and multiple organ failure.
There has been a recent increase in the geographical range of Coccidioides species and the number of reported cases owing to changes in both climate and population dynamics3. In Korea, 18 cases (including the current one) have been reported since its discovery in 19764. Of these, 11 patients had extrapulmonary involvement, and >50% of them presented with disseminated infections. Skin involve- ment was the most common (six cases), and articular, bone, and bone marrow involvement has also been reported. Brain involvement was also confirmed in two cases, including one case of mild CNS symptoms and one case of severe meningitis with blurred vision and gait disturbance5,6. Extrapulmonary infections account for only a minority of all infections (ap- proximately 1%), and disseminated infections are more common in patients of Asian ethnicity than in those living in endemic regions. They are also more common in men, children, people of non-European ethnicity, and immunocompromised individuals7,8.
Coccidioidal meningitis is a significant complication of the disseminated form of the disease. The most common symptom is headache. Other clinical manifestations may include vomiting, fever, altered levels of consciousness, or cognitive impairment. The development of hydrocephalus can confound the treatment of meningitis. Neurosurgical consultation and ventricular shunt placement may be required to control the intracranial pressure2. Coccidioidal meningitis has previously been associated with a 100% mortality rate within two years of its diagnosis. However, with the advent of intrathecal amphotericin B treatment in 1957, the mortality rate decreased by 30%9,10. Subsequently, the introduction of azoles in the 1990s revolutionized the management of coccidioidomycosis, rendering oral azoles the standard treat- ment in current practice1.
In the absence of contraindications, oral azole antifungal therapy is the standard initial treatment for almost all patients diagnosed with coccidioidal meningitis, and these patients require lifelong treatment11. Among various azole antifungal agents, fluconazole has remained the primary therapeutic option for over two decades. A daily dose of 400 mg was initially administered. However, owing to high clinical failure rates, higher daily doses ranging from 800 mg to 1,200 mg are currently commonly administered to treat coccidioidal meningitis12,13. Patients who continue to exhibit clinical mani- festations or fail to demonstrate improvement in CSF findings despite high-dose fluconazole monotherapy are administered alternative azoles or placed on intrathecal amphotericin B therapy11,14.
In the first reported case of coccidioidal meningitis in Korea, the patient experienced recurrent and worsening meningitis symptoms despite receiving maintenance therapy with azole antifungals; however, significant improvement was observed after the administration of intrathecal amphotericin B6. Our patient exhibited no improvement in impaired consciousness despite the use of fluconazole and voriconazole; thus, intra- thecal amphotericin B was considered. However, repeated EVD insertion and lumbar drainage did not affect the patient's hydrocephalus, and a local infection developed at the lumbar drainage site. Intrathecal amphotericin B could not be admin-istered to avoid the risk of exacerbating the patient's hydro- cephalus and spreading the infection through the drainage site.
Coccidioidomycosis may be effectively diagnosed by clin- ically suspecting the possibility of infection based on a history of travel or residence in an endemic area. Pulmonary coccidioidomycosis is often undetected because its initial symptoms resemble those of bacterial or viral infections2. To confirm the diagnosis, a biopsy and fungal culture can be conducted on the involved tissue; however, considerable time is required to obtain fungal culture results. Although our patient resided in Los Angeles, coccidioidomycosis was not suspected during the first visit because of the non-specificity of his systemic symptoms. Information concerning the patient's history of jogging in dusty neighborhoods was only obtained immediately before the skin biopsy, and this paucity of available information delayed the diagnosis. This patient presented with non-specific skin lesions such as ery- thematous plaques with recurrent vesicles; however, histo- pathological findings from the skin lesions provided the first indication of coccidioidomycosis, which was crucial for the diagnosis of the condition. Coccidioides species were identified in fungal cultures conducted on the skin, lung, and CSF but only after the disease had progressed to cause neurological impairment. Although antifungal treatment was initiated simultaneously with the skin biopsy, the disease had rapidly progressed to meningitis. Therefore, it was difficult to prevent further disease progression.
Herein, we report a case of coccidioidal meningitis that occurred as a complication of disseminated coccidioidomycosis invading the skin and lungs in a healthy young man with no underlying diseases. While Korea is not an endemic area for Coccidioides, its incidence has increased over the past 20 years with increased travel to high-risk areas. Delayed diagnosis of coccidioidal meningitis can be fatal even in immunocom- petent patients. Therefore, coccidioidomycosis should be suspected when patients who visit endemic areas present with lung consolidation and nodules accompanied by non-specific symptoms such as fever, myalgia, and skin lesions. Furthermore, thorough history-taking and prompt diagnosis are essential for the timely treatment and prevention of complications.
References
1. Bays DJ, Thompson GR 3rd. Coccidioidomycosis. Infect Dis Clin North Am 2021;35:453-469
Google Scholar
2. Crum NF. Coccidioidomycosis: A contemporary review. Infect Dis Ther 2022;11:713-742
Google Scholar
3. Benedict K, McCotter OZ, Brady S, Komatsu K, Sondermeyer Cooksey GL, Nguyen A, et al. Surveillance for coccidioidomycosis - United States, 2011-2017. MMWR Surveill Summ 2019;68:1-15
Google Scholar
4. Kim B, Kim N, Hwang YY, Kang HR, Shin S. Molecular identification of Coccidioides posadasii in synovial fluid in Korea. Lab Med Online 2021;11:60-63
Google Scholar
5. Lee JM, Bae SH, Lee SN, Park KH, Park CK, Yoon HK, et al. A case of disseminated coccidioidomycosis involving the lymph nodes, the skin, and the brain. Korean J Med 2012;82:734-738
Google Scholar
6. Lee JW, Kim SI, Kim YJ, Kwon JC, Lim YJ, Park MH, et al. A case of coccidioidal meningitis. Infect Chemother 2012; 44:75-79
Google Scholar
7. Wang XL, Wang S, An CL. Mini-review of published reports on coccidioidomycosis in China. Mycopathologia 2015;180:299-303
Google Scholar
8. McConnell MF, Shi A, Lasco TM, Yoon L. Disseminated coccidioidomycosis with multifocal musculoskeletal dis- ease involvement. Radiol Case Rep 2016;12:141-145
Google Scholar
9. Winn WA. The use of amphotericin B in the treatment of coccidioidal disease. Am J Med 1959;27:617-635
Google Scholar
10. Einstein HE, Holeman CW Jr, Sanddidge LL, Holden DH. Coccidioidal meningitis. The use of amphotericin B in treatment. Calif Med 1961;94:339-343
Google Scholar
11. Ho J, Fowler P, Heidari A, Johnson RH. Intrathecal ampho- tericin B: A 60-year experience in treating coccidioidal meningitis. Clin Infect Dis 2017;64:519-524
Google Scholar
12. Johnson RH, Einstein HE. Coccidioidal meningitis. Clin Infect Dis 2006;42:103-107
Google Scholar
13. Vincent T, Galgiani JN, Huppert M, Salkin D. The natural history of coccidioidal meningitis: VA-Armed Forces co- operative studies, 1955-1958. Clin Infect Dis 1993;16: 247-254
Google Scholar
14. Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treat- ment of coccidioidomycosis. Clin Infect Dis 2016;63: e112-e146
Google Scholar
Congratulatory MessageClick here!