pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Soo Hyeon Noh,Jin Kyung Chae,Sang Hyun Park,Kun Park,Eun Jung Kim
http://dx.doi.org/10.17966/KJMM.2015.20.3.70 Epub 2015 October 07
Abstract
Cutaneous mucormycosis is a rare disease caused by zygomycetes such as Rhizomucor, Mucor, Absidia, and
Rhizopus. The disease usually occurs in immunocompromised individuals, and the organism is rarely pathogenic in an immunocompetent host. Herein, we report a 77-year-old female patient who had multiple erythematous papules and pustules on the left 3rd finger. She had received systemic steroid therapy prior to the occurrence of the skin lesions. The histopathological examination of Periodic Acid Schiff stained section showed chronic granulomatous inflammation and fungal hyphae. Rhizopus species was isolated on the fungal culture of the tissue specimen. The patient was finally diagnosed with cutaneous mucormycosis and was treated with itraconazole.
Keywords
INTRODUCTION
Mucormycosis is a rare opportunistic infection caused by fungi of the order Mucorales, class Zygomycetes. Risk factors for mucormycosis include diabetic ketoacidosis, leukemia, lymphoma, cancer, AIDS, hepatitis, liver cirrhosis, anemia, congenital heart disease, burns, severe malnutrition, and iatrogenic immunosuppression[1]. According to the site of infection, there are five known types of mucormycosis, i.e., rhinocerebral, pulmonary, gastrointestinal, cutaneous, and disseminated type. The cutaneous mucormycosis is the rarest type and has the most favorable prognosis amongst the 5 types[2]. Cutaneous mucormycosis involves both epidermis and dermis, and may be caused as a primary cutaneous inoculation or secondary disseminated infection[3]. Herein, we present a case of cutaneous mucormycosis of the finger caused by Rhizopus species.
REPORT OF A CASE
A 77-year-old female presented with gradually progressive asymptomatic multiple erythematous papules and pustules on the left middle finger since 11 months. The patient had undergone systemic steroid therapy in March 18, 2014, as treatment for gout affecting her right foot. She was started on methylprednisolone 60 mg/day followed by a 6- day taper to 10 mg/day and then, the baseline dose of methylprednisolone was administered for 2 weeks.
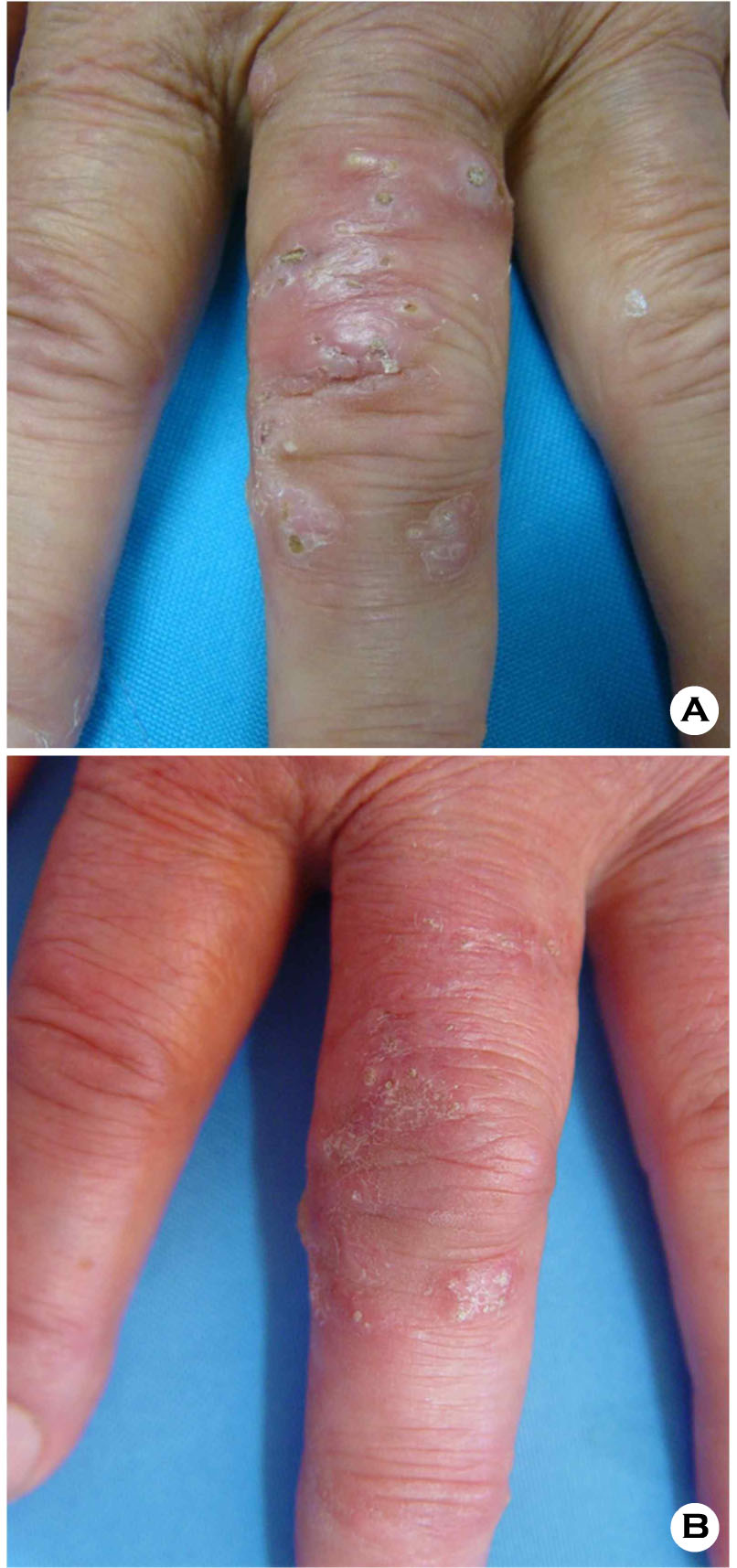
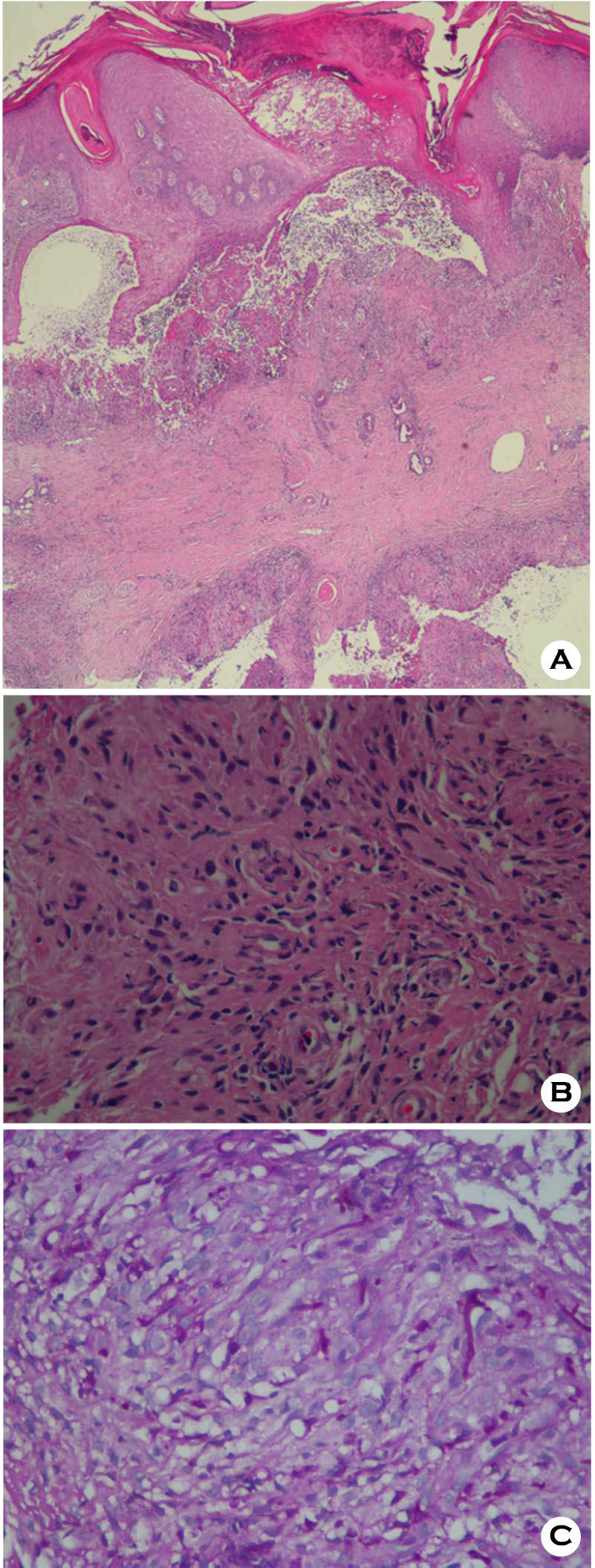
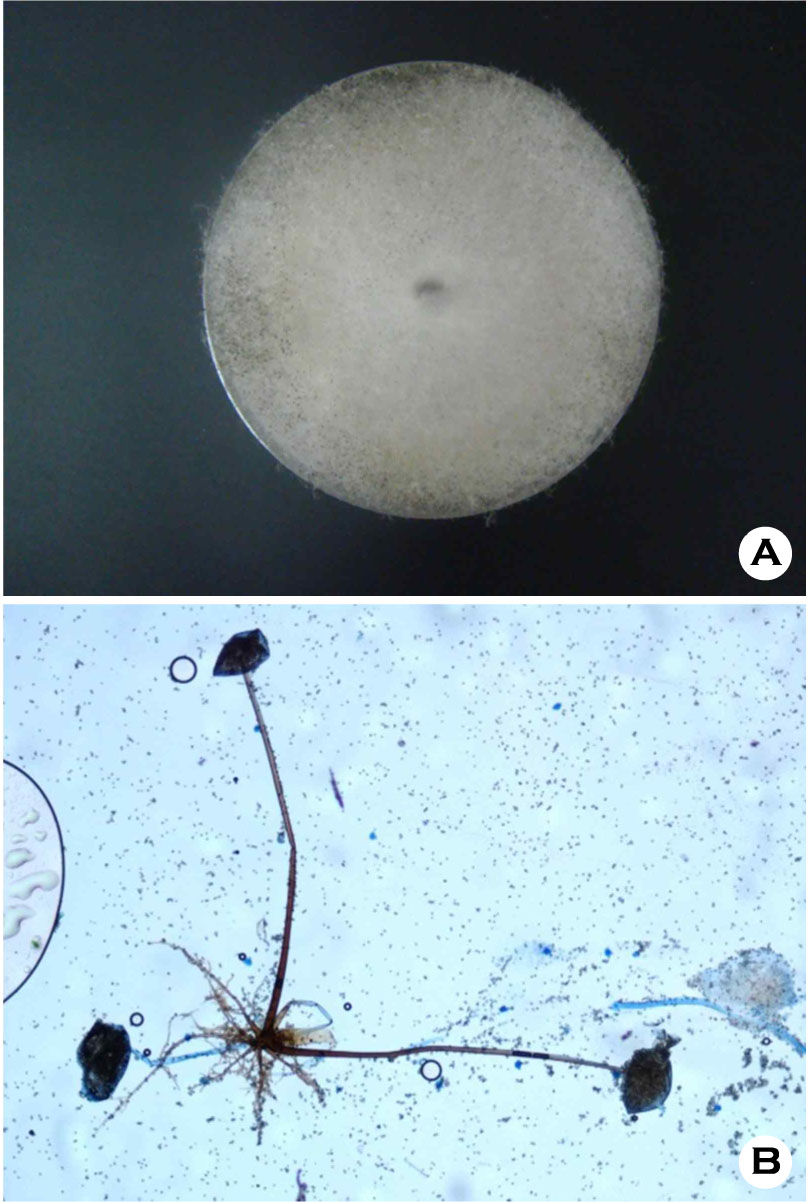
Thereafter, she was treated with methylprednisolone 5 mg/day for 2 weeks followed by 2.5 mg/day for 1 month. Approximately 1 month after administration of methylprednisolone, multiple papules and pustules with surrounding erythema occurred on the left middle finger. There was no history of trauma. She had a history of atrial fibrillation, hypertension and compression fracture of lumbar vertebrae, and had undergone bilateral subtotal thyroidectomy as treatment for goiter. Dermatological examination revealed well-defined multiple erythematous, hard papules with a central crust, and pustules on the dorsal surface of the finger (Fig. 1A). There were no signs of regional lymphadenopathy. Laboratory investigation showed a leukocyte count of 13,750/μl and hemoglobin of 11.2 g/dL. The serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 40 IU/L and 72 IU/L. The serum creatinine was 0.54 mg/dL; the blood urea nitrogen was 15.5 mg/dL. The C-reactive protein was 0.60 mg/L. Chest radiography revealed non-specific findings. Histopathology of the skin biopsy specimen under low power view showed diffuse inflammatory infiltration and necrosis in the epidermis and deep dermis (Fig. 2A). High power view of the specimen presented chronic granulomatous inflammation with lymphohistiocytes and multinucleated giant cell in the dermis (Fig. 2B). Non-septate fungal hyphae with large and long branches oriented at right angles, were observed on the microscopic examination with Periodic Acid Schiff staining (Fig. 2C). The fungal culture of the tissue specimen on Sabouraud's agar plate revealed rapidly growing whitish cottony colonies that turned dark brown at the surface of the plate (Fig. 3A). On the staining with lactophenol cotton blue stain, the fungus showed brown colored rhizoids and unbranched sporangiospores with sporangia on each end (Fig. 3B). The isolate was identified as belonging to Rhizopus species of class Zygomycetes. Based on the clinical, histopathological and mycological findings, she was diagnosed with cutaneous mucormycosis. After treatment with itraconazole (200 mg/day) for 2 months, the lesions improved with residual hyperkeratosis (Fig. 1B). The patient has been followed up for 5 months without recurrence of symptoms.
DISCUSSION
Mucormycosis is a rare fungal infection that was first reported by Paltauf in 1885[4]. The causative agent is a ubiquitous saprophytic fungus of the order Mucorales and class Zygomycetes. Nine genera are included in the order Mucorales. Rhizopus, Absidia, and Mucor are the commonly isolated genera from human tissue[2].
Cutaneous mucormycosis is categorized into two types according to the mode of infection: primary implantation of spore into the skin or secondary hematogenous dissemination from another source, especially pulmonary[5]. Primary cutaneous mucormycosis was first described by Sutherland- Campbell[6] in 1929, and is of two types[7]. The subacute superficial type is characterized by vesicles or pustules that may progress to eschar. The biopsy specimen does not show characteristic vascular invasion in this type. The gangrenous type is the more typical form and is characterized by rapidly progressive ulceration and dissemination in immunocompromised patients[7].
In the present case, the patient's skin lesions were limited to one finger and consisted of multiple papules and pustules, with no systemic signs and symptoms. Based on the findings, the following differential diagnoses were considered: deep fungal infection, dermal tuberculoid granuloma, vasculitis, and herpetic whitlow. On the skin biopsy, both epidermis and deep dermis showed chronic granulomatous inflammation and fungal hyphae. There were no unusual findings on radiological and laboratory investigation. Since Rhizopus was isol-ated on the fungal culture, she was diagnosed with the subacute superficial type of primary cutaneous mucormycosis.
Spores cause fungal infection only after overcoming the host immune response. When Rhizopus is inoculated into healthy animals, it does not induce disease because oxidative metabolites of phagocytic cells are fungicidal for Rhizopus hyphae. Besides, cationic proteins called defensins also have a fungicidal effect on Rhizopus spores and hyphae[8]. The tissue-invasive hyphae are too large to be engulfed completely by phagocytes while neutrophils have been demonstrated experimentally to impair or kill hyphae[9]. In a study involving animals with diabetes and those administered steroids, inhaled spores were shown to have invaded the lung and entered into the bloodstream. Although steroids are thought to influence the host immune system, the precise mechanism has remained largely unknown[8].
Our patient was administered systemic steroid therapy with methylprednisolone. Her treatment regimen is thought to have induced immunosuppression and the consequent infection.
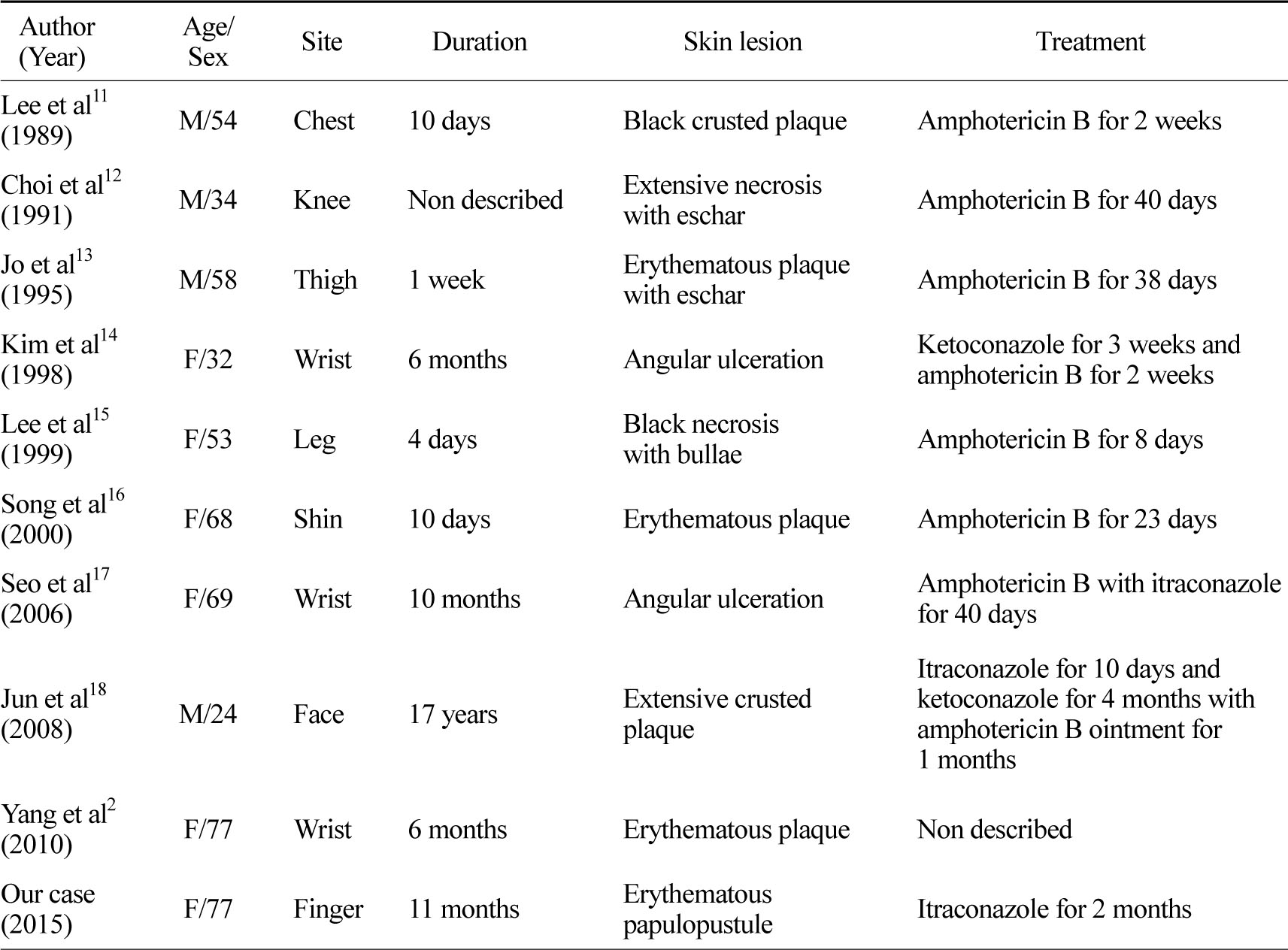
Most of the reported cases of cutaneous mucormycosis are of the life-threatening gangrenous type with amphotericin B being the treatment of choice. Liposomal amphotericin B is relatively safe at higher doses due to less nephrotoxity[10]. There are reported nine cases in Korean literatures (Table 1). But treatment duration and dosage still remain unclear[17]. And amphotericin B cannot be taken orally. Since our patient refused parenteral therapy, an orally administered alternative was chosen. One study reported successful treatment with oral itraconazole, 200 mg/day for 2 months and the patient became culturally negative[19]. However, another study found itraconazole as being unreliable against mucormycosis[10]. Since this was a case of subacute superficial cutaneous mucormycosis, which is less severe than the gangrenous type, we treated her with itraconazole monotherapy (200 mg/day). After 2 months of treatment, the skin lesions had improved with residual hyperkeratosis.
In conclusion, this case suggests that treatment with steroids or immunosuppresants can cause deep fungal infection, although its incidence has decreased with the improved infection control measures and hygienic practices for care of immunocompromised patients. In addition, the rare subacute superficial type of cutaneous mucormycosis with milder symptoms than that of the gangrenous type, is amenable to cure with itraconazole monotherapy.
Conflict of interest
The authors declare that they have no conflicts of interest in the research.
References
1. Jang HS, Moon DC, Kwon KS, Chung TA. A case of Rhinocerebral mucormycosis in a diabetic woman. Ann Dermatol 1991;3:145-152
Google Scholar
2. Yang JH, Kim SK, Lee SY, Lee JS, Park YL, Whang KU. A case of cutaneous mucormycosis mimicking sporotrichosis. Korean J Dermatol 2010;48:449-452
Google Scholar
3. Tehmeena W, Hussain W, Zargar HR, Sheikh AR, Iqbal S. Primary cutaneous mucormycosis in an immunocompetent host. Mycopathologia 2007;164: 197-199
Google Scholar
4. Paltauf A. Mycosis mucorina. Ein Beitrag zur Kenntniss der menshlichen Fadenpilzer Krankugen. Virchows Arch Pathol Anat 1885;102:543-563
5. Ameen M, Arenas R, Mrtinez-Luna E, Reyes M, Zacarias R. The emergence of mucormycosis as an important opportunistic fungal infection:five cases presenting to a tertiary referral center for mycology.Int J Dermatol 2007;46:380-384
Google Scholar
6. Sutherland-Campbell H. Paronychia:an attempt to prove the etiologic factor in an epidemic among orange workers. Arch Derm Syphilol 1929;19:233 -253
Google Scholar
7. Wirth F, Perry R, Eskenazi A, Schwalbe R, Kao G. Cutaneous mucormycosis with subsequent visceral dissemination in a child with neutropenia:A case report and review of the pediatric literature. J Am Acad Dermatol 1997;36:336-341
Google Scholar
8. Mandell GL, Bennett JE, Dolin R. Principles and practice of infectious diseases: agents of mucormycosis and related species. 4th Ed, New York, Churchill Livingstone. 1995;2311
9. Chin RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun 1982;38: 1123-1129
Google Scholar
10. Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J Jr, Ibrahim AS. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis 2009;48:1743-1751
Google Scholar
11. Lee SH, Eun HC, Lee YS. A case of cutaneous mucormycosis in a patient with acute lymphocytic leukemia. Korean J Dermatol 1989;27:440-443
Google Scholar
12. Choi YS, Woo HJ, Kang SY, Shin SW, Hong YG, Kim WJ, et al. A case of mucormycosis of skin and soft tissue in a healthy adult. Infection 1991;23:55 -59
Google Scholar
13. Jo HY, Kim CH, Kye YC, Kim SN. A case of primary cutaneous mucormycosis in a immunocompetent patient. Korean J Dermatol 1995;33:546-550
Google Scholar
14. Kim SY, Kim YJ, Chung BS. A case of cutaneous mucormycosis. Korean J Med Mycol 1998;3:200 -204
Google Scholar
15. Lee HT, Yang TH, Yoon TY, Chang SH. A considered case as primary cutaneous mucormycosis. Korean J Med Mycol 1999;4:75-78
Google Scholar
16. Song WK, Park HJ, Kim YC, Cinn YW. Primary cutaneous mucormycosis associated with trauma. Korean J Dermatol 2000;38:826-828
Google Scholar
17. Seo SH, Jang HS, Kim SJ, Kim BS, Jang BS, Kim MB, et al. Primary cutaneous mucormycosis in a immunocompetent elderly woman showing sporotrichoid distribution. Korean J Dermatol 2006;44: 1352-1356
Google Scholar
18. Jun JB, Park KD, Suh SB. Chronic recurrent cutaneous mucormycosis due to Rhizopus arrhizus. Korean J Med Mycol 2008;13:31-36
Google Scholar
19. Kumar B, Kaur I, Chakrabarti A, Sharma VK. Treatment of deep mycoses with itraconazole. Mycopathologia 1991;115:169-174
Google Scholar