pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Jung Eun Seol,Sang Woo Ahn,Seung Hee Jang,Hyojin Kim
10.17966/JMI.2023.28.1.16 Epub 2023 April 05
Abstract
Keywords
COVID-19 vaccine Lichen planus Moderna vaccine
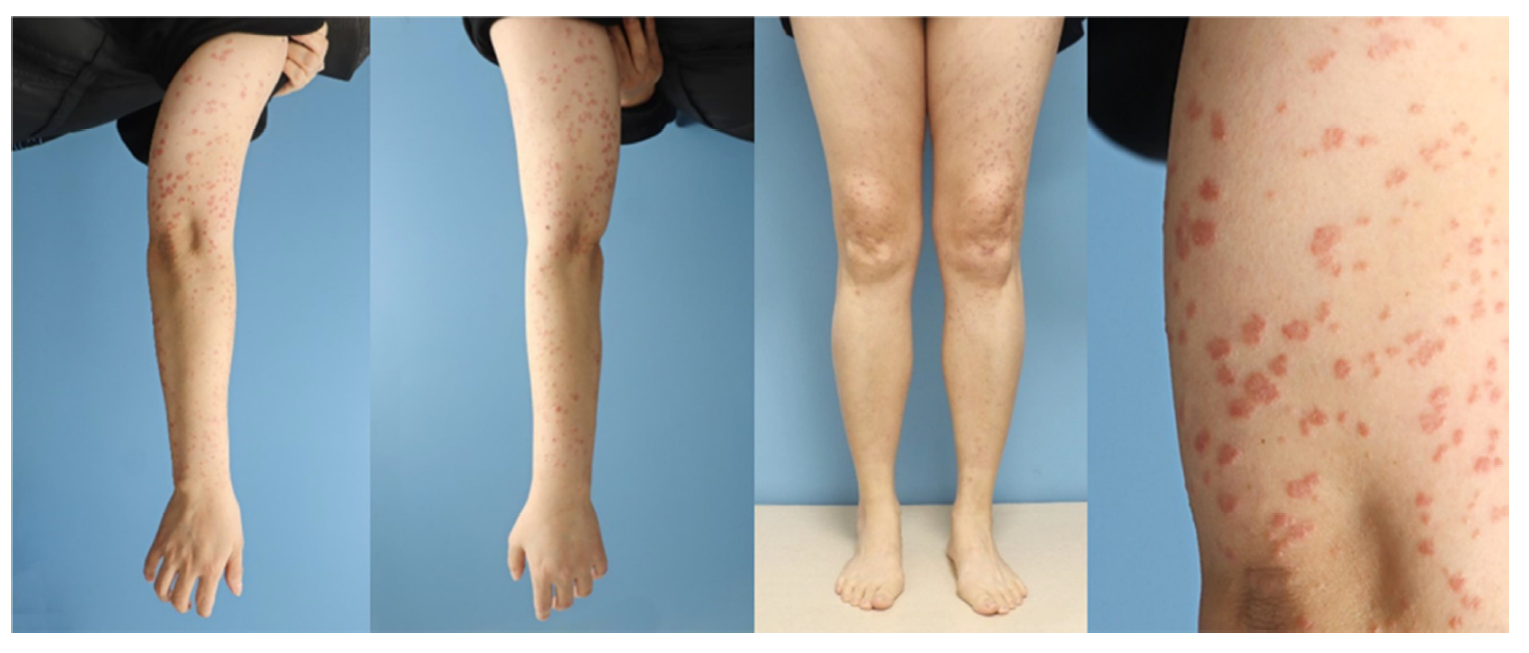
A 48-year-old woman presented with pruritic erythematous flat-topped polygonal plaques on the extensor surfaces of both extremities for 3 months (Fig. 1). The plaques developed a week after the second dose of the COVID-19 vaccine (mRNA-1273, Moderna) and did not improve with topical corticosteroid therapy. No cutaneous reaction was observed after the first dose. She had no medical or dermatological history except for stage 1A angioimmunoblastic T-cell lymph- oma on the axillae, which completely resolved with chemo- therapy 3 years ago. She denied a recent history of taking medication, COVID-19 infection, or allergic reaction. The serology test was normal for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus.
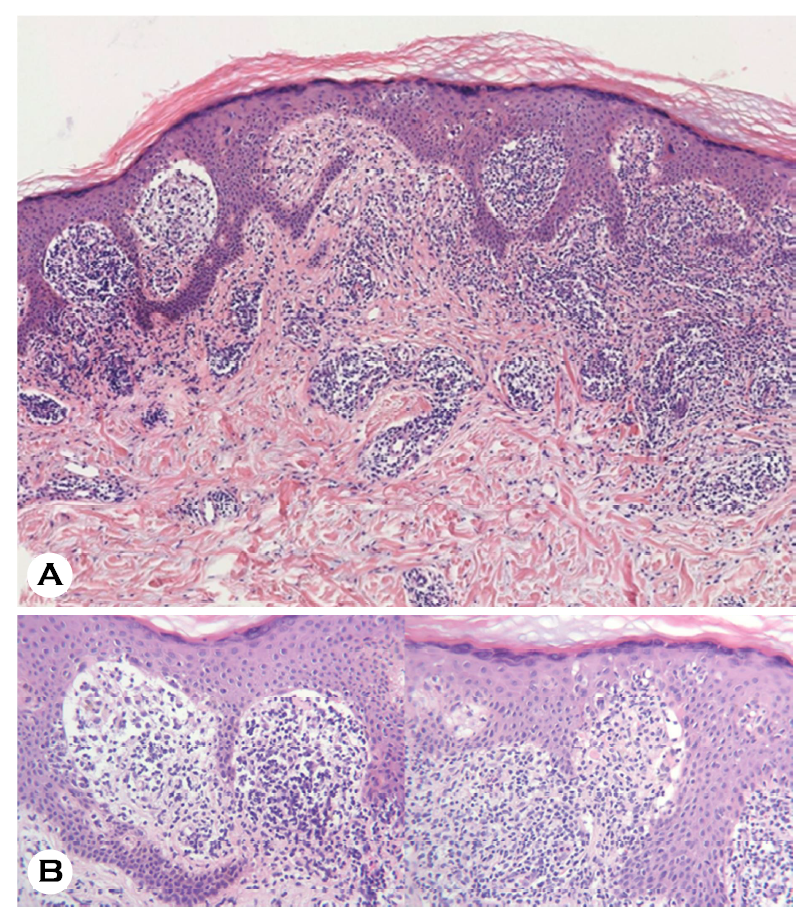
Punch biopsy of the arm revealed orthokeratotic hyper- keratosis, irregular epidermal hyperplasia, and dense band-like infiltration of basophilic cells in the superficial dermis (Fig. 2A). High-resolution magnification showed a saw-tooth appearance of the epidermis, vacuolar degeneration of basal keratinocytes, Civatte bodies, Max Joseph space, and dense infiltration of lymphohistiocytes with several melanophages (Fig. 2B). The Naranjo adverse drug reaction probability scale score was 5, suggesting "probable" level of relationship be- tween eruption and COVID-19 vaccination1. Thus, she was diagnosed with Moderna vaccine-related lichen planus (LP) and treated with oral and topical methylprednisolone, which showed a favorable response within a week without re- currence.
There are reports of variable cutaneous reactions to COVID-19 vaccines, such as urticarial, morbilliform, vesiculobullous, and papulosquamous eruptions2. In a recent systematic review, 41 cases of new onset or exacerbation of LP were found to be associated with COVID-19 vaccines3. The most common causative vaccine was Pfizer-BioNTech (41.9%), followed by Oxford-AstraZeneca (20.9%), Sinopharm (18.6%), and Moderna (9.3%)3. However, COVID-19 vaccine-related LP has not been reported in Korean literature.
A systematic review of COVID-19 vaccine-related LP by Feschuk et al.3 revealed that 41.9% of these cases developed after the second dose in our case, whereas 27.9% and 23.3% cases developed at the first dose and both doses, respectively. A similar study by Zou et al.4 showed that COVID-19 vaccine-related LP occurred at a mean age of 55.9 years with slight predominance among women (1:1.29). In both studies, proximal limbs were the most affected (45.8%), and 30.7% cases showed extensive lesions, as in our case3,4. In three cohort studies including 152 cases of COVID-19 vaccine-related LP, oral LP (96%) was more common than cutaneous LP (4%)4. Other studies showed various mani-festations such as oral LP (34.6%), classical cutaneous LP (23.1%), lichenoid drug eruption-like (15.4%), and others, including lichen planopilaris, inverse LP, and nail LP5. There was no distinguishing histopathological feature of COVID-19 vaccine-related LP from classical LP, which shows typical basal liquefaction, lichenoid lymphocyte infiltration, and apoptotic keratinocytes3-5.
The pathophysiology of COVID-19 vaccine-related LP has not been established. Immune dysregulation caused by COVID-19 spike protein antigen has been hypothesized to trigger an autoimmune reaction of CD8+ cytotoxic T-cells and B-cells against keratinocytes4. CD4+ T helper 1 cells contribute to basal keratinocyte apoptosis through cytokines, including interleukin-2, tumor necrosis factor-α, and interferon-γ, main- taining cytotoxic T cell4. Other hypotheses include type IV hypersensitivity reaction triggered by vaccine ingredients, molecular mimicry of COVID-19 antigens with human antigens on keratinocytes and cutaneous T-cell recruitment through angiotensin-converting enzyme receptor activation4,5.
References
1. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30: 239-245
Google Scholar
2. Gambichler T, Boms S, Susok L, Dickel H, Finis C, Abu Rached N, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol 2022;36:172-180
Google Scholar
3. Feschuk AM, Green M, Kashetsky N, Maibach HI. Lichen planus following SARS-CoV-2 infection and vaccination? A systematic review. Int J Dermatol 2023;62:e54-e56
Google Scholar
4. Zou H, Daveluy S. Lichen planus after COVID-19 infection and vaccination. Arch Dermatol Res 2023;315:139-146
5. Nguyen B, Perez AG, Elgart GW, Elman SA. Lichen planus after COVID-19 infection and vaccination: A systematic review. J Eur Acad Dermatol Venereol 2023;37:e278-e281
Congratulatory MessageClick here!