pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Jin Hee Kim,Ji Hyun Lee,Joonsoo Park,Hyun Chang Ko,Jong Soo Choi,Hyojin Kim,Jee Bum Lee,Jin Park,Beom Joon Kim,Yang Won Lee
10.17966/JMI.2021.26.2.35 Epub 2021 July 01
Abstract
Background: Onychomycosis is a common nail fungal infection that affects the nail bed, plate, and matrix. It accounts for ~50% of all nail diseases. The treatment of onychomycosis includes systemic and topical approaches, which depend on patient compliance, drug penetration and delivery to the nail bed, antifungal efficacy, and tolerance. Efinaconazole is a novel triazole antifungal agent that is active against many pathogenic fungi, and is used for treating onychomycosis.
Objective: To confirm the efficacy and safety of 10% efinaconazole solution for treating onychomycosis.
Methods: This study was conducted as a 52-week, multicenter, open, single-group, phase 4 clinical trial. A 10% efinaconazole solution was applied on the toenails of each subject before bedtime, once a day for 48 weeks. The subjects visited the test facility every four weeks for the evaluation of the efficacy and safety of the treatment.
Results: At 52 weeks of applying 10% efinaconazole solution, the rates of complete cure, mycological cure, complete or almost complete cure, and clinical effectiveness were 16.7%, 95.8%, 38.54%, and 52.1%, respectively. The clinical effects of efinaconazole were seen in more than half of the subjects. No severe adverse reactions related to efinaconazole were observed.
Conclusion: The antifungal effect and safety of topically applied 10% efinaconazole solution in patients with mild to moderate toenail onychomycosis caused by dermatophytes was confirmed based on the efficacy and safety evaluation for a complete cure.
Keywords
Dermatophyte Efinaconazole Onychomycosis Toenail
Onychomycosis is a common fungal infection of nails that affects the nail bed, plate, and matrix. It accounts for ~50% of all nail diseases. Various types of fungi easily infect the nail apparatus. Because of the slower growth rate of toenails than that of fingernails, toenail onychomycosis occurs seven to ten times more frequently compared with fingernail onychomycosis. A typical lesion of onychomycosis is characterized by distal subungual keratosis with an irregular border toward the nail bed, which progresses over time. Sometimes, a yellow streak in the proximal direction and onycholysis may occur, leading to permanent destruction and deformation of toenails, which seriously affects the quality of life (including concerns regarding appearance) and hinders wearing of shoes, walking, and participating in various sports activities.
The most common causative agents of onychomycoses are dermatophytes, particularly Trichophyton rubrum and T. mentagrophytes var. interdigitale, whereas Epidermophyton floccosum, T. violaceum, and T. soudanense are rare causative agents. In warm climates, Candida albicans can cause a clinically identical nail infection due to the presence of enzymes that can degrade keratin. Although other yeast species may be found in nail infection, their pathogenic role is unclear. The recurrence rate after successful treatment of onychomycoses ranges from 11.9% to 33.7%.
Although onychomycoses can be diagnosed clinically, confirmation via direct microscopy, after treating the subungual- keratotic debris with 10~20% potassium hydroxide, culturing, and histopathological examination, is required. The rate of false-negative results, particularly for cultures, is high (between 30% and 60%). In contrast, histopathological diagnosis has been proved to be doubly sensitive.
Recent onychomycosis treatments include systemic and topical approaches, the success of which depends on patient compliance, drug penetration and delivery to the nail bed, antifungal efficacy, and tolerance. Slow toenail growth and the ability of clinicians to measure the clinical efficacy of the drug quickly are essential to effective treatment. In general, oral treatments have been used for onychomycosis. However, the potential of drug interactions in some patients, especially the elderly who receive many concomitant medications, limits the treatment options due to safety concerns (such as liver toxicity) and the need for potential monitoring.
Only itraconazole and terbinafine, with complete cure rates of 14% and 38%, respectively, are approved for treating onychomycosis in the United States. However, topical treatment is preferred because oral treatment has potential systemic side effects and requires laboratory monitoring. Although local side effects of topical therapy are not serious compared with the possible adverse reactions associated with oral therapy, the reported cure rate of topical therapy is much lower than that of systemic therapy. The treatment period is longer (6~12 months). Ciclopirox nail lacquer has a modest effect in mild to moderate onychomycosis, with a complete cure rate of 5.5~8.5%. However, frequent nail surface debridement is required when using this product.
Efinaconazole is a novel triazole antifungal agent that is effective against various pathogenic fungi. It is applied as an external solution and is used for treating onychomycosis. In particular, it exhibits in vitro efficacy against dermatophytes (Trichophyton, Microsporum, and Epidermophyton species), and yeast (Malassezia species, C. albicans, and other Candida species). Efinaconazole external solution has low affinity for keratin and high reversibility compared with other reference drugs. As a topical treatment for onychomycosis, efinaconazole is applied on nails and the surrounding skin to deliver the primary component, and thus nail debridement is not required.
Previously, efinaconazole external solution has been tested in clinical trials for toenail onychomycosis. Its efficacy was evaluated in phase 2 (once a day for 3 weeks), and phase 3 (once a day for 48 weeks) clinical trials. In a pivotal phase 3 test, it was consistently and statistically superior to the vehicle regarding all the primary and secondary efficacy endpoints (p<0.0001). Moreover, in phase 3 clinical trials conducted in the United States, Japan, and Canada, efinaconazole consistently exhibited higher efficacy than that of the vehicle, regardless of age, sex, ethnicity, race, or median-infected toenail area. In a phase 3 clinical trial, 1,640 patients with onychomycosis used efinaconazole or vehicle once a day for 48 weeks, and 2,763 adverse reactions were reported. Most of the individually reported cases occurred in a relatively small number of subjects (<1% in each treatment group), and similar rates were reported in the efinaconazole group (65.3%) and vehicle group (59.8%). There was no change in clinical test parameters over time, as well as in the number of patients with normal values at screening and that of patients with abnormal values at subsequent study visits. There was no clinically significant difference between efinaconazole, vehicle, and individual clinical test results reported as adverse reactions. When applied to the toenail once a day as a topical solution, the permeation of efinaconazole resulted in low systemic concentration levels. Overall, in addition to the ability of the patient to drive or work with machines, there were no concerns about the safety of efinaconazole related to overdose, drug abuse, discontinuation, or rebound effects. In this context, we conducted a phase 4 clinical trial to confirm the safety and effectiveness of efinaconazole application in Korean subjects.
This was a 52-week, multicenter, open, single-group, phase 4 clinical trial. The patients were subjected to a screening test (Visit 1) within 6 weeks before starting the application of the test drug (Visit 2, baseline), and the individuals adjudged to meet the selection criteria at the time of screening and baseline evaluation (Visit 2) were included. A registration number was given to each patient, and the trail was conducted. Efinaconazole 10% topical solution was applied on the toenails once a day before bedtime for 48 weeks. Subjects visited the test facility every 4 weeks and were evaluated for efficacy and safety of the treatment. Nine investigators from nine Korean institutions participated in this study. The trial was approved by the Institutional Review Board of each institution and was conducted according to international scientific/ethical standards. Informed consent was obtained from each subject.
1. Subjects
The subjects included adult males and females, aged 19 years or older, with a positive direct potassium hydroxide test for the target nail, those with mild to moderate onychomycosis (with an infected area of 20%, or more, and 50% or less), and those with positive fungal culture test for dermatophyte or dermatophyte/Candida.
We excluded the subjects who had fungal spikes within 3 mm of the proximal nail fold of the target nail or had fungal infection only in the lateral part of the target nail; who were diagnosed with one-hand two-foot syndrome or severe moccasin tineapedis; who had a surgical history of the target nail before participating in the clinical trial; who had a history of immunosuppression, immunodeficiency disease, or had clinical signs related to an immunosuppressive disease (based on the judgment of the investigator), or were infected with the human immunodeficiency virus; with uncontrolled diabetes; with other diseases that may cause nail abnormalities or influence the evaluation of investigational drugs, based on the judgment of the investigator; who had a history of hypersensitivity to azole derivatives or components of efinaconazole external solution; or with a history of not responding to systemic antifungal drugs previously administered for the treatment of onychomycosis.
The efficacy data obtained in this clinical trial were analyzed in full (FA), set, and per protocol (PP) sets. In principle, the FA set was used for the primary analysis, whereas the PP set was used for additional analyses. Safety analysis was conducted using the safety set.
1) FA set
It included subjects who were given the drug once or more after registration in the clinical trial. Patients in this group were evaluated for the primary efficacy endpoint once after drug application.
2) PP set
The PP set included the subjects who completed every scheduled visit without any protocol violation (listed below).
(i) Violation of selection/exclusion criteria
(ii) In case of other serious violations of the clinical trial protocol, efficacy evaluation, application method related to primary efficacy evaluation, or visit date.
3) Safety sets
It included subjects who received the drug at least once after registration, and in whom the safety-related data were evaluated at least once, either via the telephone or during a visit after application.
2. Drug application
The subjects applied topical 10% efinaconazole solution once a day (for 48 weeks) before bedtime on all onychomycosis infected toenails. The solution was applied on the nail fold, nail bed, hyponychium under the nail plate, and surface under the nail plate. The first application was per- formed during the baseline visit (Visit 2) in the laboratory after a detailed explanation of the application method to the subjects. The application site was allowed to dry completely before it came in contact with bed sheets, socks, or clothing.
3. Efficacy and safety evaluation
1) Primary efficacy endpoint
The primary efficacy endpoints had a complete cure rate at 52 weeks after application, the number of subjects with 0% infected area, and those who showed mycological cure. Mycological cure was defined as a negative result in both potassium hydroxide microscopy and fungal culture examinations.
2) Secondary efficacy endpoints
The secondary efficacy end points were as follows:
(1) Complete or almost complete cure rate at 52 weeks after application (the percentage of subjects with <5% infected area of the target nail and showing mycological cure).
(2) Mycological cure rate at 52 weeks after application (the number of subjects showing negative results in both direct potassium hydroxide microscopy and fungal culture examinations of the target nail).
(3) Clinical effectiveness rate at 52 weeks after application (the percentage of subjects with <10% infected area of the target nail).
3) Safety evaluation
Subjects were evaluated at each visit for adverse reactions, subjectively, or by the investigator. Vital signs and clinical laboratory tests were referenced.
Safety evaluation of adverse reactions was classified into three groups—mild, moderate, and severe—as follows:
(1) Mild adverse reaction: Recognizable symptoms were observed; however, the severity of these symptoms is mild enough not to cause any harm in daily life activities.
(2) Moderate adverse reaction: Symptoms that require treatment because of an adverse reaction that interferes with daily life.
(3) Severe adverse reaction: Symptoms are too severe that daily life activities cannot be performed.
4. Statistical analysis
This clinical trial evaluated the antifungal effects and safety of topical efinaconazole solution based on descriptive data analysis. For statistical testing, a two-sided test was conducted at a significance level of 5%. The amount of subjects, average, standard deviation, median, minimum, and maximum values are presented as descriptive statistics for continuous data, whereas the frequency and ratio are presented as descriptive statistics for categorical data. When statistical tests were conducted for changes before and after application, the satisfaction of the prenormality assumption for continuous data was confirmed on the bases of Shapiro-Wilk test. When the normality assumption was satisfied, a paired t-test was conducted, and when it was not enough, a Wilcoxon signed rank test was conducted to confirm the statistical significance. All changes in data before and after application satisfied normality and were compared using a paired t-test. Changes in categorical data before and after application were examined for statistical significance by conducting the McNemar's test. The method for processing the calculated data and the data expression format, including the time of evaluation, are detailed in the statistical analysis plan. The analysis was conducted using SPSS version 25.0 software for Windows (IBM®SPSS®, USA).
1. Participants
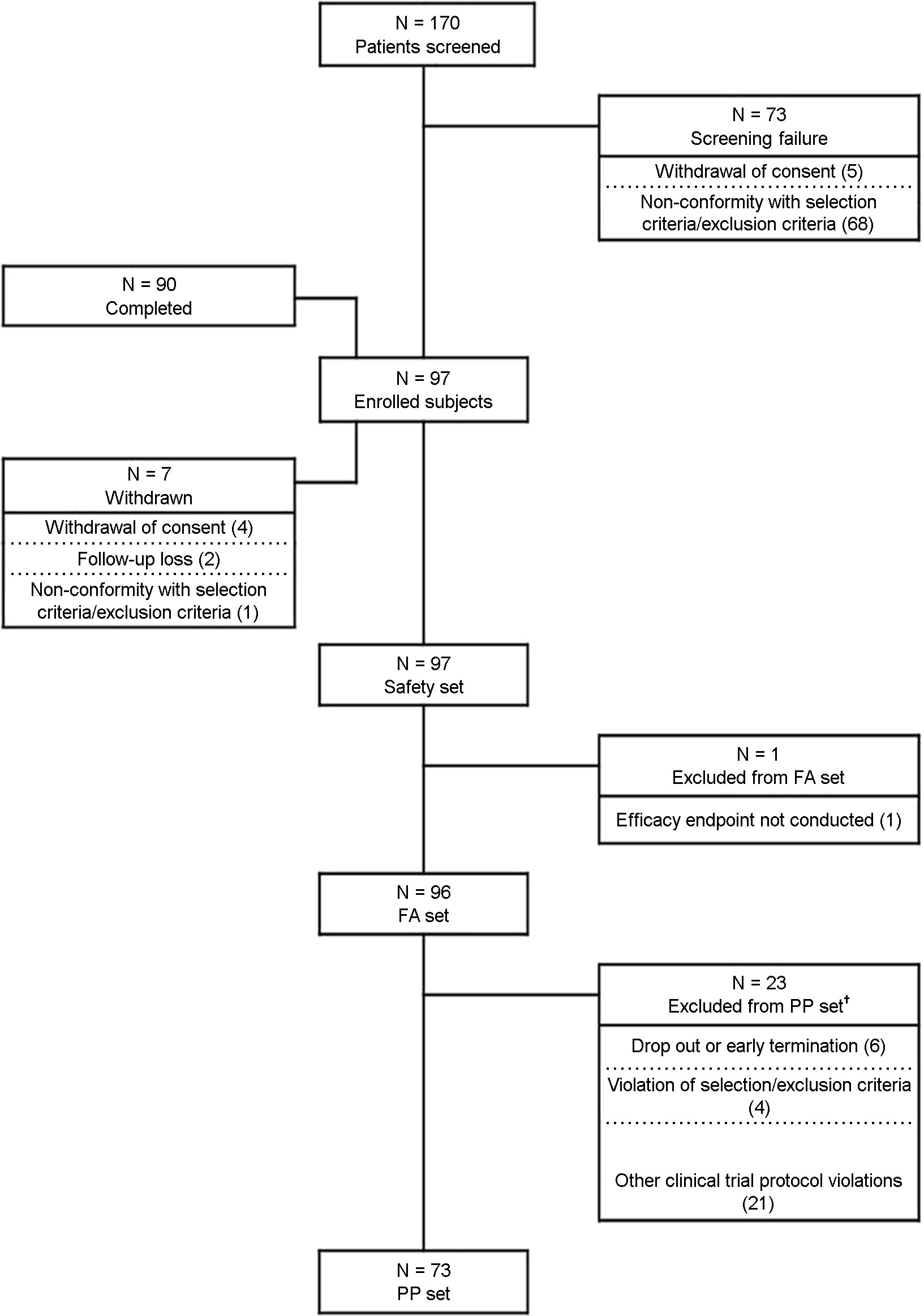
The clinical trial was conducted at nine institutions in the Republic of Korea. Total 170 subjects were screened with their consent. Among them, 97 subjects were enrolled in the clinical trial, whereas 73 were excluded because they with- drew consent or did not meet the selection criteria. Among the registered subjects, seven subjects, including four who withdrew the consent, two who did not come for the next visit or lost contact, and one who did not meet the selection criteria, were dropped. Ninety patients completed the clinical trial. Fig. 1 shows the detailed participation status of the subjects in the trial.
The subjects included 71 males and 26 females, with an average age of 52.5±12.8 years. The average height was 168.1±7.7 cm, and the average weight was 69.2±11.5 kg. No pregnant female subject was tested. Detailed demographic information is presented in Table 1.
|
|
|
Enrolled subjects |
|
Sex |
Male, n (%) |
71 (73.20) |
|
|
Female, n (%) |
26 (26.80) |
|
Age |
n |
97 |
|
|
Mean ± SD |
52.51±12.76 |
|
|
Median |
54.00 |
|
|
Min-Max |
24.00~82.00 |
|
Height (cm) |
n |
97 |
|
|
Mean ± SD |
168.11±7.68 |
|
|
Median |
168.00 |
|
|
Min-Max |
150.00~188.00 |
|
Weight (kg) |
n |
97 |
|
|
Mean ± SD |
69.15±11.48 |
|
|
Median |
68.90 |
|
|
Min-Max |
48.20~106.00 |
Before administering efinaconazole solution, the average toenail length, thickness, and infected area were 5.36±3.56 mm, 2.07±0.74 mm, and 36.32%±9.01%, respectively. In the fungal culture test, T. rubrum and T. mentagrophytes were cultured in 90 and 6 subjects, respectively. Detailed information on the preapplication characteristics of the target nail is presented in Table 2.
|
Target nail (Baseline) |
|
Enrolled subjects |
|
Toe
nail length (mm) |
n |
97 |
|
|
Mean ± SD |
5.36±3.56 |
|
|
Median |
4.00 |
|
|
Min-Max |
3.00~22.00 |
|
Thickness
of toe nail |
n |
97 |
|
|
Mean ± SD |
2.07±0.74 |
|
|
Median |
2.00 |
|
|
Min-Max |
1.00~3.00 |
|
Infected
area (%) |
n |
97 |
|
|
Mean ± SD |
36.32±9.01 |
|
|
Median |
35.00 |
|
|
Min-Max |
20.00~50.00 |
|
Fungal
culture |
|
|
|
Trichophyton
rubrum |
n (%) |
90 (93.75) |
|
Trichophyton
mentagrophytes |
n (%) |
6 (6.25) |
|
Microsporum |
n (%) |
0 (0.00) |
|
Candida |
n (%) |
0 (0.00) |
|
Other dermatophytes |
n (%) |
0 (0.00) |
2. Treatment compliance
At each visit, unused efinaconazole solution prescribed during the previous visit was collected. The compliance was evaluated based on the number of applications recorded in the diary by the subject. The average treatment compliance was 96.00%±8.02% (minimum, 40.27%; maximum, 100.00%) for 96 subjects in the FA set, and 97.26%±4.45% (minimum, 82.49%; maximum, 100.00%) for 73 subjects in the PP set (Table 3).
|
Treatment |
|
FA set |
PP set |
|
Total |
n |
96 |
73 |
|
|
Mean ± SD |
96.00±8.02 |
97.26±4.45 |
|
|
Median |
99.40 |
99.70 |
|
|
Min-Max |
40.27~ |
82.49~ |
|
†Treatment
compliance (%) = (actual number of doses)/ (total number of doses) × 100, total number of doses = [last date of administration (48
weeks ± 5 days) or
premature discontinuation
date] - first date of administration |
|||
3. Efficacy evaluation
For the FA set, the complete cure rate at 52 weeks after application (the primary efficacy analysis for the target group) was 16.77% (16/96 subjects), and 95% bilateral correct confidence interval was between 9.84% and 25.65%. At 12 and 24 weeks after application, the complete cure rate was both 1.04% (1/96); 2.08% (2/96) at week 36 and 8.33% (8/96) at week 48. The values for 95% bilateral correct confidence interval for the complete cure rate at 52 weeks for the FA set and the complete cure rate after application are presented in Fig. 2.
For the PP set, the complete cure rate at 52 weeks after application was 17.81% (13/73), and 95% bilateral correct confidence interval was between 9.84% and 28.53%. At 12 weeks after application, the complete cure rate was 1.37% (1/73); however, one subject for whom complete cure was confirmed at week 12, did not visit for a follow-up, and no subject expressed complete cure at week 24. The complete cure rate was 2.74% (2/73) at week 36, and 9.59% (7/73) at week 48. The 95% two-sided correct confidence interval for the complete cure rate at 52 weeks for the PP set and the complete cure rate after application are presented in Fig. 2.
For the FA set, the mycological cure rate at 52 weeks after application was 95.83% (92/96), and the 95% bilateral correct confidence interval was between 89.67% and 98.85%. The mycological cure rate at each visit was 63.54% (61/96) at week 12, 94.79% (91/96) at week 24, 93.75% (90/96) at week 36, and 97.92% (94/96) at week 48. In the PP set, the mycological cure rate at 52 weeks after application was 95.89% (70/73), and the 95% bilateral correct confidence interval was between 88.5% and 99.1%; the mycological cure rate was similar to that for the FA set.
The clinical effectiveness rate was defined as the proportion of subjects in whom the infected area of the target nail was 10% or less after efinaconazole application. The clinical effectiveness rate at 52 weeks after efinaconazole application was 52.08% (50/96) in the FA set, and the 95% bilateral correct confidence interval was between 41.64% and 62.39%. Clinical effects were observed in more than half of the sub- jects. The clinical effectiveness rate was 2.08% (2/96) at week 12, 10.42% (10/96) at week 24, 21.88% (21/96) at week 36, and 42.71% (41/96) at week 48. The clinical effectiveness rate at 52 weeks after application was 58.90% (43/73) in the PP set. A 95% two-sided correct confidence interval was between 46.77% and 70.29%, thereby showing a similar result for the FA set.
In the FA set, the complete cure rate at 52 weeks after application, according to the age group, was the highest at 33.33% (3/9) in subjects aged 70 years or above, followed by 26.32% (5/19) in subjects aged 40~49 years, 20.00% (1/5) in 19~29 year group, 20.00% (6/30) in 50~59 year group, and 7.69% (1/13) in 30~39 years group. For the PP set, the complete cure rate at 52 weeks after application, according to age group, was the highest at 35.71% (5/14) in subjects aged 40~49 years, 33.33% (1/3) in 19~29 years group, 28.57% (2/7) in subjects aged 70 years or above, 20.00% (4/20) in 50~59 years group, and 9.09% (1/11) in 30~39 year group. The complete cure rate at 52 weeks after application was 17.65% (3/s17) and 13.33% (2/15) for subjects aged 65 years or above and 16.46% (13/79) and 18.97% (11/58) for those aged <65 years, for the FA and PP sets, respectively.
Analysis of a complete cure rate at 52 weeks after application in subjects with or without concomitant diseases revealed that in the FA set, none of the 11 subjects with diabetes showed a complete cure, and 18.82% (16/85) of the subjects without diabetes showed a complete cure. A complete cure was confirmed in 10.00% (1/10) of the subjects with liver function diseases, whereas 17.44% (15/86) of the subjects without liver function diseases showed a complete cure at 52 weeks. Among the seven subjects with diabetes in the PP set, none showed a complete cure at 52 weeks after efinaconazole application. In contrast, 19.70% (13/66) of the subjects without diabetes showed a complete cure, with a complete cure confirmed in 14.29% (1/7) and 18.18% (12/ 66) of the subjects with and without liver function diseases, respectively.
Analysis of the complete cure rate at 52 weeks after efina- conazole application in the target nail revealed that 17.78% (16/90) of the subjects infected with T. rubrum in the FA set expressed complete healing, and none of the six subjects infected with T. mentagrophytes was completely cured. In the PP set, among the subjects infected with T. rubrum, 18.84% (13/69) showed a complete cure, whereas none of the four subjects infected with T. mentagrophytes showed a complete cure.
In the FA set, analysis of the complete cure rate at 52 weeks after application, according to the infection area of the target nail at the baseline, revealed that the percentage of subjects with severe (40~50%), mild (20~29%), and moderate (30~ 39%) infection showing a complete cure was 25.00%, (10/ 40), 20.00% (4/20), and 5.56% (2/36), respectively. In the PP set, the percentage of subjects showing complete cure was 27.27% (9/33) in the severe, 15.38% (2/13) in mild, and 7.41% (2/27) in moderate infection groups.
In the FA set, analysis of the complete cure rate at 52 weeks after application, according to the target nail thickness at the baseline, revealed that a complete cure was observed in 21.74% (5/23) of the subjects with nail thickness of 1 mm or less, 18.18% (8/44) with nail thickness >1 mm and <2 mm, and 10.34% (3/29) with thickness of >2 and <3 mm. In the PP set, a complete cure was observed in 20.00% (4/20) of subjects with thickness of <1 mm, 19.44% (7/36) with thickness >1 and <2 mm, and 11.76% (2/17) with thickness >2 and <3 mm.
4. Safety evaluation
The average period of application of efinaconazole solu- tion was 329.8±47.3 days (range, 6~396 days). The average number of application was 317.9±55.1 (range, 4~396). Among the 97 subjects included in the safety set, 132 cases of adverse reactions were reported in 50 (51.55%) subjects who received efinaconazole treatment. Infections and in- festations exhibited the highest frequency, with 44 cases in 27 patients (27.84%), and nasopharyngitis (14 cases in 11 patients; 11.34%) was the most frequent infection. Among the observed adverse effects, 100 cases were mild, 31 were moderate, and one was severe. In order of frequency of occurrence, six cases in five patients (5.15%) were mild nasopharyngitis, and eight cases in six patients (6.19%) were of moderate nasopharyngitis, whereas no case with serious severity was observed. Regarding headache, a nervous system disorder, there were four mild cases in four patients (4.12%), but no moderate and severe cases. There were no mild and moderate urinary calculus (a renal and urinary disorder) and one case of severe urinary calculus (1.03%). All the reactions were identified as adverse reactions unrelated to efinaconazole application.
There were six cases of severe adverse reactions in three patients (3.09%), and the severity was moderate. None of the adverse reactions involved efinaconazole. Adverse events of special interest included 14 cases in 10 patients (10.31%), all of which were of mild severity, not serious adverse events and not related to efinaconazole. During the trial period, there were no adverse drug reactions, adverse reactions that resulted in death or caused treatment discontinuation.
Hematology and blood chemistry tests revealed that hemoglobin was normal before application (Visit 1) but was found to be abnormal at 48 weeks after application (Visit 14) in five subjects (5.49%), and the change was statistically significant (p=0.0253). Clinically significant abnormalities were not observed in any subject. Among the vital signs (systolic blood pressure, diastolic blood pressure, and pulse), blood pressure exhibited a statistical significant increase after the application of efinaconazole. At baseline (Visit 2), the average blood pressure was 120.88±9.98 mmHg, whereas at 48 weeks after application (Visit 14), it was 123.27± 9.02 mmHg (p=0.0367).
Efinaconazole (molecular formula, C18H22F2N4O; molecular weight, 348.39), synthesized as an azoleamine derivative, was approved by the FDA in the US for topical treatment of toenail onychomycosis caused by T. rubrum and T. mentagrophytes. It blocks the biosynthesis of ergosterol, which maintains the fluidity of fungal cell membranes, creates a permeability barrier, and is essential for fungal cell viability.
Efinaconazole inhibits ergosterol biosynthesis in both T. mentagrophytes and C. albicans. It causes concentration-dependent changes in the morphology of T. mentagrophytes hyphae. It is an inhibitor of 14α-demethylase in the ergosterol pathway and causes degenerative changes. Because of its low affinity for keratin, the primary component of nails, efinaconazole solution has superior nail permeability and antifungal activity in the nail.
Here, we evaluated the antifungal efficacy and safety of efinaconazole external solution, administered for 48 weeks, in patients with mild to moderate toenail onychomycosis caused by dermatophytes. The complete cure rate at 52 weeks after efinaconazole application in the FA set was 16.67% (16/96 patients), and the 95% correct confidence interval for the complete cure rate was between 9.84% and 25.65%. The complete cure rate at the time of additional analysis was 1.04% (1/96) at 12 and 24 weeks, 2.08% (2/96) at 36 weeks, and 8.33% (8/96) at 48 weeks.
In phase 3 studies of efinaconazole 10% solution, complete cure rates at 52 weeks were 17.8% (Study 1) and 15.2% (Study 2). In this clinical trial, the complete cure rate at 52 weeks after the application of efinaconazole was 16.67%, which was similar or higher than that reported in phase 3 clinical trials of efinaconazole 10% solution.
Among the secondary efficacy endpoints, complete or nearly complete cure rate at 52 weeks after application was 38.54% (37/96) in the FA set, and the 95% bilateral correct confidence interval was between 28.78% and 49.03%. At 52 weeks after application, the mycological cure rate in the FA set was 95.83% (92/96), and the 95% bilateral correct confidence interval was between 89.67% and 98.85%. At 52 weeks after efinaconazole application, the clinical effec- tiveness rate was 52.08% (50/96), with clinical effects seen in more than half of the subjects, and the 95% correct con- fidence interval for both sides was between 41.64% and 62.39%. Although the cure rate was similar or superior to those reported in previous studies, the mycological cure rate was higher (95.83%) than those reported in previous clinical studies (approximately 50~60%). Comparing the negative rate in the fungal culture test, both the present and previous studies showed a cure rate of 97~98%. However, there was a significant difference in the results of the negative rate of potassium hydroxide microscopy examination (98.9%) com- pared with a study conducted in Japan (57.6%) and those conducted in the US and Canada (54.7%). The negative rate in potassium hydroxide microscopy examination in this study was similar to the negative rate in the fungal culture test. This indicated that potassium hydroxide microscopy examination test, a relatively subjective test, showed high proficiency and accuracy in this study.
To compare the complete cure rates among the elderly in the FA set, the subjects were divided into two age groups: 65 years or above and below 65 years. The complete cure rate was higher in subjects aged 65 years or above (17.65%, 3/17) than that of those aged below 65 years (16.46%, 13/79); however, the difference was not statistically significant. In patients over 70 years of age, the highest complete cure rate was 33%; however, the results were not statistically significant due to the small number of subjects. Therefore, a study with a larger number of subjects is warranted in the future.
At 52 weeks after application, with or without comorbid diseases, none of the 11 subjects with diabetes showed complete cure, whereas 18.82% (16/85) of subjects without diabetes showed complete cure. However, there were no significant differences between the two groups. In line with previous studies, there was no relationship between the presence or absence of diabetes and the effect of efinazonazole.
T. rubrum, which accounts for 50% of dermatophytes, is an essential target for treating onychomycosis. Among the 90 subjects in whom T. rubrum was identified in the target nail at the baseline, 16 (17.78%) showed complete healing. In contrast, none of the six subjects infected with T. menta- grophytes exhibited complete cure. There was no significant difference between the two groups.
Moreover, there was no statistical difference in the complete cure rate according to the thickness of the baseline target nail or the area of infection. The results of our study were consistent with previous studies, which reported that efinaconazole was effective in patients with onychomycosis, regardless of severity, the affected area, and age, including severe cases.
No serious adverse reactions related to efinaconazole were observed, and there was no dropout in the trial because the drug was maintained in all subjects with adverse reactions. Thus, efinaconazole was confirmed to be safe.
In clinical laboratory tests, systolic blood pressure increased from an average of 120.88±9.98 mmHg at the baseline to 123.27±9.02 mmHg at 48 weeks after application. Significant changes were observed; however, considering that no blood pressure related adverse reactions were reported, the results were not clinically meaningful. There was no significant difference in diastolic blood pressure and pulse.
In a previous phase 3 study wherein efinaconazole was administered for 48 weeks, in grown toenail, application site dermatitis, application site vesicles, and application site pain were reported as adverse effects, accounting for 1% or more of adverse reactions. In this clinical trial, ingrown nail and dermatitis were each reported in 1.03% (1/97, 1 case) subjects, and both the cases were of mild severity and not related to efinaconazole.
Onychomycosis is a common fungal infection of nails that is difficult to treat successfully. The antifungal effect and safety of topical 10% efinaconazole solution were confirmed in patients with mild to moderate toenail onychomycosis, which is caused by dermatophytes, based on our analysis of the complete cure rate.
References
1. Beuscher TL, Kelechi TJ. Onychomycosis: Diagnosis, treat- ment, and prevention. J Wound Ostomy Continence Nurs 2019;46:333-335
2. Piraccini BM, Sisti A, Tosti A. Long-term follow-up of toenail onychomycosis caused by dermatophytes after successful treatment with systemic antifungal agents. J Am Acad Dermatol 2010;62:411-414
Google Scholar
3. Cuchi-Burgos E, Rubio-Casino R, Ballestero-Tellez M, Pariente-Jimenez F, Perez-Jove J, Blanco-Suarez A. Com- mercial real time PCR implementation for rapid diagnosis of onychomycosis: A new workflow in a clinical laboratory. Enferm Infecc Microbiol Clin 2020
Google Scholar
4. Gupta AK, Foley KA, Mays RR, Shear NH, Piguet V. Monotherapy for toenail onychomycosis: a systematic review and network meta-analysis. Br J Dermatol 2020; 182:287-299
Google Scholar
5. Bhatt V, Pillai R. Efinaconazole topical solution, 10%: formulation development program of a new topical treatment of toenail onychomycosis. J Pharm Sci 2015; 104:2177-2182
Google Scholar
6. Elewski BE, Rich P, Pollak R, Pariser DM, Watanabe S, Senda H, et al. Efinaconazole 10% solution in the treat- ment of toenail onychomycosis: Two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol 2013;68:600-608
Google Scholar
7. Rodriguez RJ, Low C, Bottema CD, Parks LW. Multiple functions for sterols in Saccharomyces cerevisiae. Biochim Biophys Acta 1985;837:336-343
Google Scholar
8. Parks LW, Smith SJ, Crowley JH. Biochemical and physio- logical effects of sterol alterations in yeast--a review. Lipids 1995;30:227-230
Google Scholar
9. Tatsumi Y, Nagashima M, Shibanushi T, Iwata A, Kangawa Y, Inui F, et al. Mechanism of action of efinaconazole, a novel triazole antifungal agent. Antimicrob Agents Che- mother 2013;57:2405-2409
Google Scholar
10. Gupta AK, Studholme C. Update on efinaconazole 10% topical solution for the treatment of onychomycosis. Skin Therapy Lett 2016;21:7-11
Google Scholar
11. Noguchi H, Matsumoto T, Hiruma M, Asao K, Hirose M, Fukushima S, et al. Topical efinaconazole: A promising therapeutic medication for tinea unguium. J Dermatol 2018;45:1225-1228
Google Scholar
12. Iozumi K, Abe M, Ito Y, Uesugi T, Onoduka T, Kato I, et al. Efficacy of long-term treatment with efinaconazole 10% solution in patients with onychomycosis, including severe cases: A multicenter, single-arm study. J Dermatol 2019;46:641-651
Google Scholar
Congratulatory MessageClick here!