pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed

pISSN : 3058-423X eISSN: 3058-4302
Open Access, Peer-reviewed
Jae Young Sung,Ji Won Lim,Chang Yong Kim,Young Ah Cho,Yang Won Lee,Yong Beom Choe ,Da-Ae Yu
10.17966/JMI.2023.28.4.117 Epub 2024 January 05
Abstract
Diabetes mellitus (DM) is a metabolic disorder that causes chronic hyperglycemia, leading to immune dysfunction and increased vulnerability to infections. As a result, patients with DM are more susceptible to fungal infections, including Candida species, due of their weakened immune systems. Cutaneous candidiasis is a fungal infection that appears as a red plaque with peripheral satellite papules and pustules around the periphery. Patients with chronic mucocutaneous candidiasis may have erythematous plaques and thickened skin thickening with an overlying scale, which can mimic plaque psoriasis. Therefore, fungal infections should be considered when assessing chronic refractory psoriasiform lesions that are difficult to treat, especially in patients with DM. This report describes a case of cutaneous candidiasis in both lower legs, which was initially misdiagnosed as psoriasis.
Keywords
Cutaneous candidiasis Diabetes mellitus Psoriasiform candidiasis
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia that may produce long-term com- plications like retinopathy, neuropathy, and nephropathy1. Several immune alterations have been described in patients with diabetes, including progressive worsening of cellular immunity2. Thus, patients with DM are more prone to fungal infections than healthy individuals. Cutaneous candidiasis is a common infection associated with DM3 that manifests as bright red plaques that can be erosive, dry, scaly, oozing, or macerated; pustules and collarette scales may also be present4. Clinically, cutaneous candidiasis lesions may show morpho- logies similar to those of other diseases, such as psoriasis, characterized by well-demarcated, thick, scaly, erythematous plaques, which could confuse clinicians. Here, we report a case of cutaneous candidiasis in the lower legs of a patient with uncontrolled DM.
A 67-year-old woman visited the hospital with complaints of painful, scaly plaques and crusts on both her lower legs, along with a one-year history of severe nail dystrophy (Fig. 1). Her past medical history was significant for DM, hypertension, and hyperlipidemia. The lesions first appeared as 5-6 ery- thematous papules on her left foot after receiving her first COVID-19 vaccination. After the second dose, she developed additional erythematous scaly papules and plaques on the left foot, and new annular erythematous lesions appeared on the right foot. She was diagnosed with an allergic reaction to the vaccine at a local clinic and was prescribed oral and topical steroids. However, after four months of treatment, the lesions became painful and began to ooze. Another local clinic diagnosed her with psoriasis and prescribed topical steroids; however, the lesions did not improve and she was referred to the dermatology department.
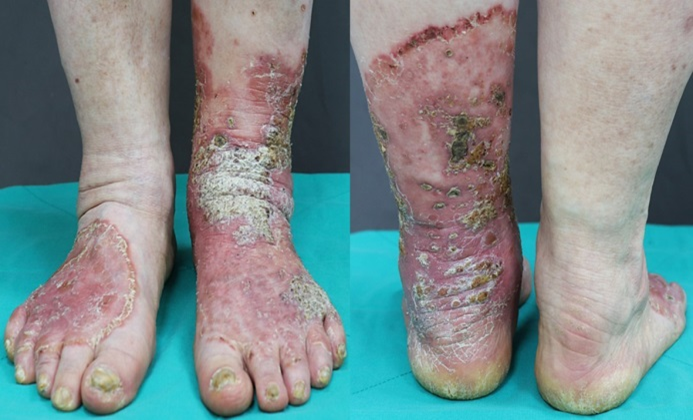
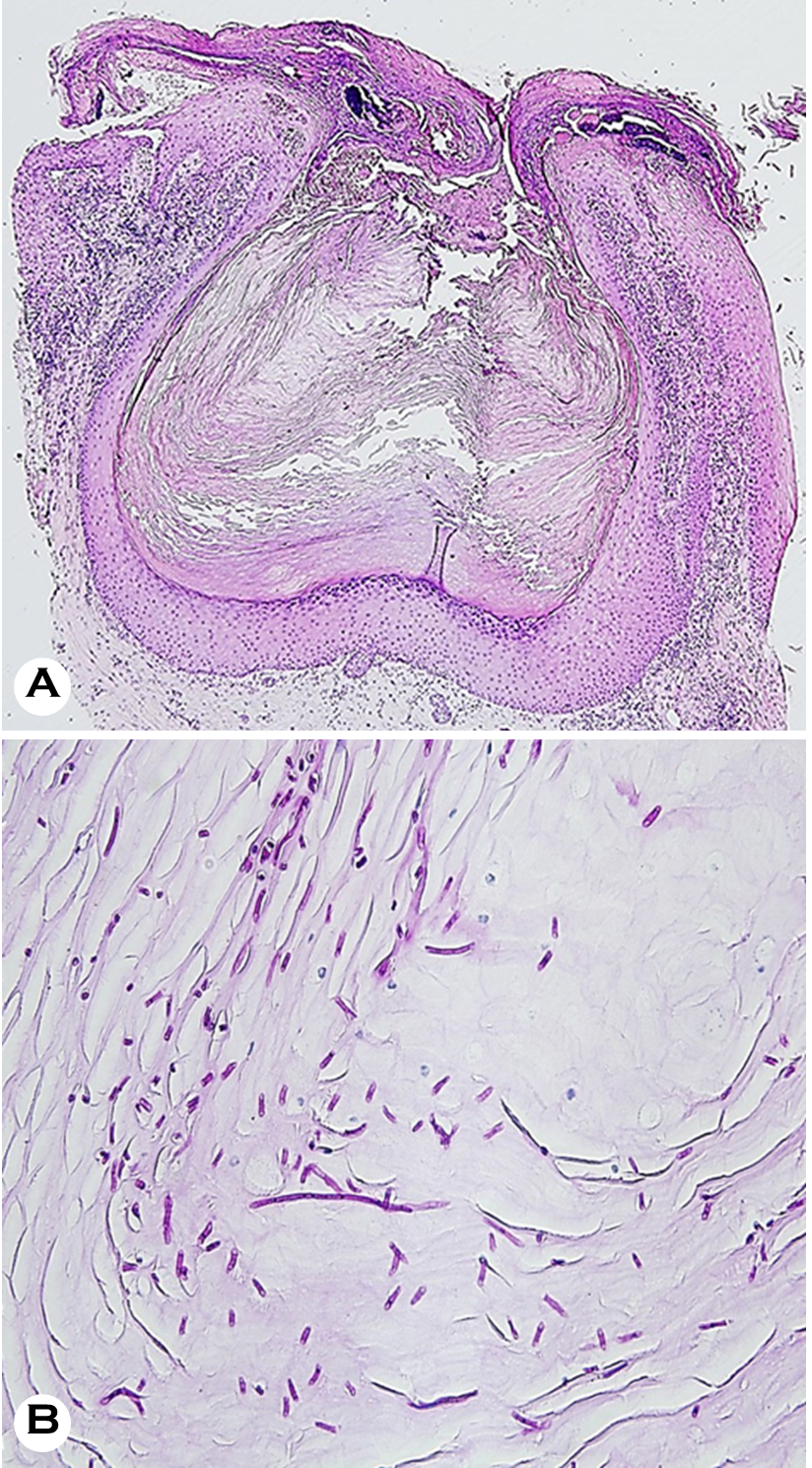
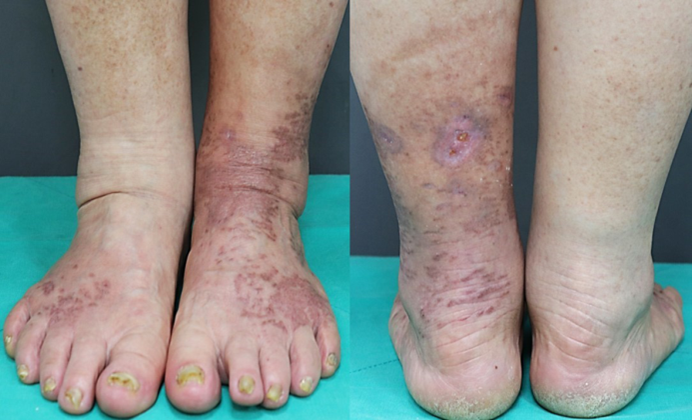
On assessment, she had wide-spread painful, demarcated erythematous scaly plaques and crusts on both feet and lower legs and severely dystrophic toenails. No abnormal findings were observed during the system review. Blood tests showed elevated blood glucose (250 mg/dL; normal values: 70~99 mg/dL) and HbA1c levels (10.7%; normal range: 4.3~5.6%). The results of the complete blood count, liver function test, and renal function test results were all within normal limits. With the initial impression of plaque psoriasis and fungal infection, a KOH smear, fungal culture, and skin biopsy were performed. The KOH smear revealed numerous hyphae, confirming superficial fungal infection. Skin biopsy with H&E and D-PAS staining showed follicular plugging and crusts with numerous fungal spores and hyphae, which implied Candida infection rather than dermatophyte infection (Fig. 2). The fungus cultured from skin lesions isolated from Candida albicans. The final diagnosis was chronic cutaneous candidiasis, for which antifungal treatment with oral itra- conazole (200 mg daily) and topical amorolfine cream twice daily for 5 weeks. Unfortunately, the lesions did not improve substantially, and the treatment was changed to oral flu- conazole 150 mg weekly with topical sertaconazole cream for 5 weeks. After switching to fluconazole, her skin and nails started to significantly improve (Fig. 3).
People with DM are susceptible to various skin diseases, from infectious to autoimmune5. This is because they experi- ence prolonged hyperglycemia and insulin deficiency, which makes them more vulnerable to infections, neuropathy, suppression of cytokine production, defective phagocytosis, immune cell dysfunction, and a suboptimal immune response against bacteria6. Patients with DM also have a higher like- lihood of developing cutaneous fungal infections due to their high blood sugar levels, with a relatively high incidence of tinea pedis and onychomycosis 5.
Candida yeasts are common in the environment and can be found on human skin and in the oropharyngeal, respiratory, gastrointestinal, and genital areas9. These yeasts can cause various infections, from mild mucosal or skin issues to severe, life-threatening, and invasive infections7. Candida skin in- fections are quite common, affecting people of all ages and accounting for about 1% of all outpatient visits to dermato- logical clinics and 7% of all inpatient visits8. Some of the most common fungal pathogens that cause skin and nail infections include Candida albicans and Candida tropicalis11. Typically, the infection begins with a pruritic rash that contains vesiculopustules, which then enlarge and often progress to maceration and erythema. The affected area has a scalloped border with a white rim of necrotic epidermis surrounding the erythematous macerated base, and satellite lesions are frequently observed11. Candida folliculitis is mainly found in hair follicles and rarely becomes extensive11. While all body regions may be involved, the most common areas affected are intertriginous areas, such as the groin folds, abdominal skin folds, inframammary folds, and interdigital spaces4.
Cutaneous candidiasis is a fungal infection that affects the skin. While it can be treated with antifungal medication, its clinical presentation can be similar to that of other skin conditions, leading to delayed diagnosis and treatment. Mis- diagnosis of cutaneous candidiasis occurred in a case of cutaneous candidiasis that mimicked acute generalized exan- thematous pustulosis (AGEP). A 67-year-old male with mul- tiple comorbidities presented with pruritic pustular eruptions and fever (38.2℃). Because he was previously treated with a 7-day course of antibiotics for bacteremia, the initial diagnosis was AGEP. However, a KOH examination and fungal culture revealed a cutaneous Candida infection, and the patient responded well to topical and oral antifungals. Zhang et al.10 reported in the case of an otherwise healthy 21-year-old male who presented with facial cutaneous candidiasis, which manifested as acne-like lesions, including papules, comedones, pustules, and cysts on both cheeks. He was initially diagnosed with acne; however, the lesions worsened despite treatment with oral minocycline. Because the fungal culture revealed C. albicans, topical and oral antifungals were prescribed, and the skin lesions improved. Patients with chronic mucocutaneous candidiasis may present with erythematous scaly plaques that resemble plaque psoriasis9.
Current treatments for cutaneous candidiasis typically in- volve topical and oral therapies with anti-inflammatory, anti- bacterial, and antifungal effects8. A systematic review of treat- ment strategies found that topical clotrimazole, nystatin, and miconazole were equally effective8. Fluconazole is currently the only evidence-based option for systemic treatment8.
Skin lesions caused by candidiasis can often resemble other skin diseases, making diagnosing difficult. If misdiagnosed and treated with steroids, it can further complicate the con- dition and delay proper treatment. Therefore, conducting a complete examination and taking a detailed medical history is crucial to ensure a correct and prompt diagnosis, especially in patients with multiple underlying health issues.
References
1. Willis AM, Coulter WA, Fulton CR, Hayes JR, Bell PM, Lamey PJ. Oral Candidal carriage and infection in insulin-treated diabetic patients. Diabet Med 1999;16:675-679
Google Scholar
2. Calvet HM, Yoshikawa TT. Infections in diabetes. Infect Dis Clin North Am 2001;15:407-421
Google Scholar
3. Gosh K, Das K, Gosh S, Chakraborty S, Jatua SK, Bhattacharya A, et al. Prevalence of skin changes in diabetes mellitus and its correlation with internal diseases: a single center observational study. Indian J Dermatol 2015;60:465-469
Google Scholar
4. Armstrong AW, Bukhalo M, Blauvelt A. A Clinician's guide to the diagnosis and treatment of candidiasis in patients with psoriasis. Am J Clin Dermatol 2016;17:329-336
Google Scholar
5. Surya SP, Dewi KP, Regina R. Cutaneous candidiasis mimicking inverse psoriasis lesion in a type 2 diabetes mellitus patient. J Gen Proced Dermatol Venereol Indones 2020;5:56-61
6. 6. Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its impact on the immune system. Curr Diabetes Rev 2020;16:442-449
Google Scholar
7. Seale E, Spurr A, Teo I, Glassman SJ. Cutaneous candi- diasis mimicking acute generalized exanthematous pus tulosis: A case report. SAGE Open Med Case Rep 2023; 11:2050313X231160949
8. Taudorf EH, Jemec GBE, Hay RJ, Saunte DML. Cutaneous candidiasis - an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol 2019;33:1863-1873
Google Scholar
9. Ahronowitz I, Leslie K. Yeast Infections. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, et al., editors. Fitzpatrick's Dermatology. 9th ed. New York: McGraw-Hill, 2019:2952-2959
10. Zhang W, Yu X, Yao Z. Case of acne-like lesion of cutaneous candidiasis in a healthy man. Int J Dermatol 2020;59:e148-e150
11. Vazquez JA, Sobel JD. Candidiasis. In: Kauffman CA, Pappas PG, Sobel JD, Dismukes WE editors. Essentials of Clinical Mycology. 2nd ed. New York: Springer Science, 2011:167-175
Congratulatory MessageClick here!